Effect of alectinib versus crizotinib on progression-free survival, central nervous system efficacy and adverse events in ALK-positive non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Lung cancer is the most common malignant tumor, and it remains the major cause of cancer-related death worldwide (1). Based upon the microscopic appearance of tumor cells, lung cancers are classified into two main types: small cell lung cancer (15–20%) and non-small cell lung cancer (NSCLC) (80–85%) (1). Anaplastic lymphoma kinase fusion gene-rearrangement (ALK-positive) NSCLC is a unique subgroup that accounts for 3–7% of NSCLC cases (2). The gene rearrangement occurs on chromosome 2 and increases the tyrosine kinase energy of the ALK receptor, stimulating proliferation and tumor survival. Advanced NSCLC associated with the ALK fusion oncogene is highly sensitive to ALK tyrosine kinase inhibitors (3). Over the last few years, the introduction of several ALK inhibitors has completely altered the treatment of advanced ALK-positive NSCLC and significantly improved the prognosis for patients (4).
Crizotinib was the first ALK inhibitor developed, and it has demonstrated systemic efficacy and strongly improved outcomes in NSCLC patients with ALK-positive when compared with chemotherapy. Compared with standard chemotherapy, crizotinib greatly prolongs progression-free survival (PFS). However, many NSCLC patients with ALK-positive (5,6) experience clinical progression and frequent brain metastasis within the first year of treatment with crizotinib (7). The predisposition toward central nervous system (CNS) progression during crizotinib treatment is attributed to poor accumulation of the drug in the CNS, since this drug can be readily effluxed from the CNS through P-glycoprotein (P-gp)-mediated transport. Therefore, CNS penetration is crucial in combating resistance after first-line crizotinib treatment. It is imperative to develop next-generation ALK inhibitors that can overcome acquired drug resistance and CNS activity.
Alectinib (CH5424602; Chugai Pharmaceutical and F. Hoffmann-La Roche, Basel, Switzerland) is an efficient and selective second-generation ALK inhibitor (8). Alectinib was designed specifically to be a more potent and selective anti-ALK therapeutic agent that could bypass crizotinib resistance (9). Alectinib is effective in vitro in treating several crizotinib-resistant mutations in ALK, including L1196M, F1174L, R1275Q, and C1156Y (10). L1196M is considered the gatekeeper mutation in crizotinib-resistant mutants, and alectinib can selectively inhibit the growth of L1196M-driven tumors (11-13). Alectinib shows high antitumor activity in ALK-positive (11,14,15), crizotinib-naive or crizotinib-resistant NSCLC patients (including patients with CNS metastases). Data from 1,251 patients in Japan who received 300 mg twice daily alectinib confirm that alectinib has a favorable safety and effectiveness profile in NSCLC patients with ALK-positive (16). Two randomized Phase III trials compared alectinib with crizotinib in patients with previously untreated, advanced ALK positive NSCLC, including those with asymptomatic CNS disease (J-ALEX, n=207 patients; ALEX, n=303 patients). Patients who received alectinib had significantly longer PFS than those who received crizotinib in these trials (11,12). Two single-arm, phase II studies [NP28673, global (NCT01801111) and NP28761 (17), North American (NCT01871805)] demonstrated that Alectinib [600 mg twice daily (BID)] had excellent efficacy and acceptable safety in NSCLC patients with ALK-positive after crizotinib failure (15). In a phase III J-ALEX study (12), alectinib also showed greater clinical efficacy and better tolerance than crizotinib. Therefore, alectinib showed significant improvements in PFS and tolerability compared with crizotinib. In addition, alectinib can retain a higher concentration in the CNS and effectively reduce brain metastases.
Although alectinib has shown impressive results, its short history has left it underused, and its precise efficacy, especially side effects, still lacks sufficient understanding. This paper aims to evaluate the efficacy and side effects of alectinib through meta-analysis and hopes to help clinicians use alectinib more appropriately.
Methods
Search strategy
This analysis was carried out according to statement guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (18). We searched four electronic databases: PubMed, EMBASE, Cochrane and Web of Science. The following terms were used in the search: ((alectinib[Title/Abstract]) AND (crizotinib[Title/Abstract]) AND ((non-small cell lung cancer[Title/Abstract]) OR (NSCLC[Title/Abstract])) AND ((PFS) OR (progression-free survival) OR (progression-free survival) OR (central nervous system))). The cut-off date of the search was 30 April 2019. References of identified articles were also manually retrieved to assess potentially eligible studies. The scope of inclusion was not limited by language of publication. All abstracts were screened twice, and unrelated studies were excluded.
Selection criteria
Two authors (Yanli Yang and Ruolan Xiang) independently screened titles and abstracts to identify potentially eligible articles. Then, they checked the full text to determine final inclusions according to the inclusion and exclusion criteria. Reasons for exclusion were documented. The authors settled differences by jointly reviewing the study in question. If no consensus was reached, a third reviewer (Jinghua Yang) functioned as an arbiter. If there were multiple reports of the same trial, we included data from the most up-to-date reference. Case reports, reviews, comments, editorials, letters or articles unrelated to our topics were excluded. All articles that met the following prespecified PICOS criteria were deemed eligible and were included. P: patients with NSCLC; I: treated with alectinib; C: treated with crizotinib or without control; O: PFS, cumulative incidence of CNS progression, incidence of adverse events (AEs); and S: randomized controlled trials (RCTs) or cohort study.
Data extraction and analysis
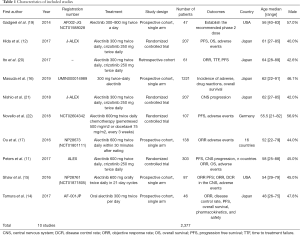
Two authors (Yanli Yang and Ruolan Xiang) independently extracted data from each included study using predefined extraction forms. For the extraction forms, we designed a standard sheet with the following variables: (I) first author; (II) year of publication; (III) study type and study design; (IV) population and number of patients; (V) treatment arms; and (VI) endpoint outcomes (Table 1). Differences among authors were resolved by discussion and consensus. Study quality was researched independently by two authors (Yanli Yang and Ruolan Xiang), and any difference was resolved by a joint evaluation of the original article.
Full table
Definition of outcomes
To explore the efficacy and safety of alectinib, we calculated the following: (I) the hazard ratio (HR) to compare alectinib-related and crizotinib-related PFS; (II) pooled estimates of cumulative incidence of CNS progression in patients treated with alectinib at the 6th and 12th months and 95% confidence interval (CI); and (III) combined incidence of 28 cases of AE grade ≤2 and 9 cases of AE grade ≥3.
Assessment of study quality and risk of publication bias
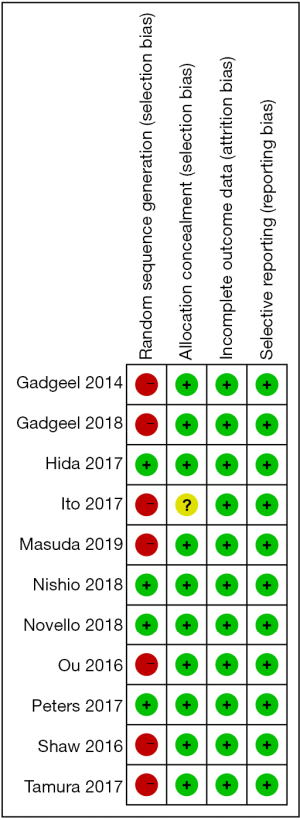
The quality of the studies was assessed using the Cochrane Risk of Bias tool (version 5.1.0, The Nordic Cochrane Centre, Denmark). Items were scored as low, high or unknown risk of bias. Two investigators assessed the risk of bias in the included studies independently, and disagreements were resolved via consensus of the two reviewers or by arbitration by a third investigator as necessary. Since all clinical trials were open-labeled, no blind assessment was performed.
Publication bias was assessed using the Begg rank correlation test and the Egger weighted linear regression test. When possible publication bias was observed, the trim-and-fill method was used to estimate the number of missing studies not published, augment the data to make the funnel plot more symmetrical, and calculate a summary estimate based on the augmented data.
We performed the sensitivity analysis using the method of “removing one study”.
Statistical methods
The inverse variance method and a fixed-effects model were used to analyze PFS and test the heterogeneity (I2) of different studies. The Dersimonian and Laird pooled model (D + L model) was used to calculate a pooled cumulative incidence of CNS progression and a pooled incidence of AEs. All analyses were performed in STATA (Version 14; Stata Corp. College Station, Texas, USA).
Results
Selection of eligible studies
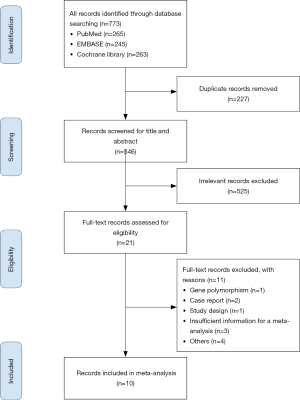
A total of 773 studies were identified by screening abstracts, and duplicates were excluded. After 546 studies were selected for second screening, 21 studies were assessed by full-text review and qualification evaluation. Ten studies were eventually included in this meta-analysis. Among them, 4 studies were RCTs, and 6 studies were prospective or retrospective cohort studies. Figure 1 shows a flow chart of the search selection in this meta-analysis.
Characteristics of included studies
After the eligibility assessment, 10 studies comprising 2,377 patients were included, and the characteristics of the included trials are illustrated in Table 1. Ten articles seem like a small number, but it has only been a few years since the approval of alectinib by the US FDA in December 2015, and there are few studies that can meet our meta-analysis goals. Among the 4 RCTs, Hida et al. (12) and Nishio et al. (21) were from the same J-ALEX trials. The reason we included these 2 studies based on the same trials is that their data complement each other (the data of PFS came from Hida et al., and the data of CNS progression came from Nishio et al.); another RCT, Novello et al. (22), had a chemotherapy control group instead of the crizotinib therapy group, and we extracted data only from its alectinib therapy group. One retrospective study, Ito et al. (20), whose population inclusion criteria and treatment were similar to those of J-ALEX, was included, and we extracted alectinib-related versus crizotinib-related PFS. Furthermore, 5 single-arm prospective cohort studies were included to analyze the cumulative incidence rate of alectinib in CNS progression and the incidence rate of AEs. RCT’s single-arm cumulative incidence rate of alectinib in CNS progression and the incidence rate of AEs were also used and combined with data from 5 single-arm prospective cohort studies. For evaluation of study quality, see the Cochrane Risk of Bias tool evaluation form (Figures 2,3).

PFS of alectinib versus crizotinib
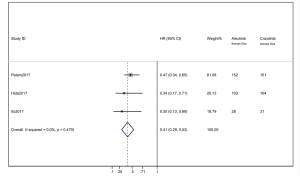
We combined the HR of PFS from the 3 studies. Alectinib showed significant PFS superiority over crizotinib. The pooled HR =0.41 (95% CI: 0.29–0.53) indicated that the alectinib therapy group did have significantly longer PFS than that of the crizotinib group. The heterogeneity was 0 (I2=0%) (Figure 4). When the “one by one removal” method was used, there was no change to the combined effect, and the sensitivity of a single clinical trial was low.
Cumulative incidence of CNS progression of alectinib
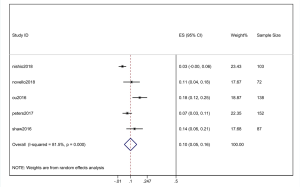
Five studies (3 RCTs and 2 prospective cohort studies) were included to generate the cumulative incidence of CNS progression following alectinib treatment at two timepoints (the 6th and 12th month). For the 3 RCTs, only alectinib treatment data were analyzed. As the percentage of CNS metastases at baseline was different, there was risk of heterogeneity across the 5 selected studies. We used points on the diagram to acquire accurate data if the data could not be directly provided by the study.
The pooled cumulative incidence of CNS progression in patients treated with alectinib at the 6th month was 10% (95% CI: 5–16%), and there was heterogeneity (I2=81.5%) (Figure 5); at the 12th month, it was 16% (95% CI: 9–24%), and there was heterogeneity (I2=85.8%) (Figure 6). A random effects model was used because of the high heterogeneity. We performed a “one by one removal” method to observe the effect of each study on the combined effect value and found no sensitive studies. The cumulative incidence of CNS progression of alectinib treatment at the 12th month was twofold higher than the value at the 6th month reported in the study by Nishio et al. (21) and Novello et al. (22). In the 3 other clinical trials, the cumulative incidence of CNS progression of the latter 6 months was much lower than that of the former 6 months (11,15,17).
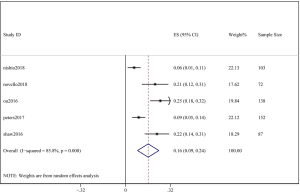
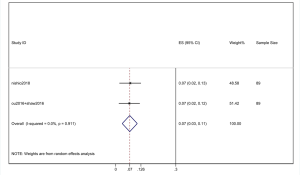
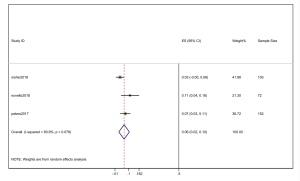
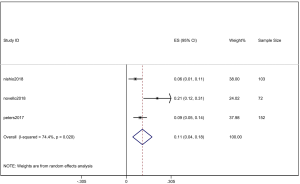
However, the percentage of CNS metastases at baseline is different, and the pooled results cannot accurately reflect the effect of alectinib on CNS progression. Therefore, we combined the results from those studies in which there was no CNS metastases at baseline before alectinib treatment. Nishio et al., 2017 provided the cumulative incidence of CNS progression in patients without baseline CNS metastases; however, Show et al., 2016 and Ou et al. did not provide this indicator. Gadgeel et al., 2018 reported an exploratory analysis study that provided a combined CNS incidence of the above two studies. The pooled incidence of CNS progression at 12 months was 7% (95% CI: 3–11%) in patients with no baseline CNS metastases from the 3 trials (Figure 7). It is worth noting that in two studies, Novello et al., 2017 and Nishio et al., 2017, the incidence of CNS progression in patients without baseline CNS metastasis was 0 at 6 months; further, Ou et al., 2016 and Shaw et al. 2016 reported an incidence rate of 5.7%. Since the value 0 could not be merged, we only combined the incidence rate at 12 months. The incidence of CNS progression in patients without baseline CNS metastases was significantly lower than in patients with baseline CNS metastases mixed with patients without baseline CNS metastases. In addition, due to the differences between the study nature of RCTs and cohorts, we performed a stratification analysis. We combined the cumulative incidence of CNS progression of RCTs only. The incidence of CNS progression at 6 and 12 months was 5% (95% CI: 2–10%) and 11% (4%, 18%), respectively. The heterogeneity decreased; I2 was 60.9% and 74.4%, respectively (Figures 8,9).
Assessment of AEs of alectinib
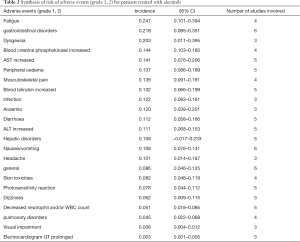
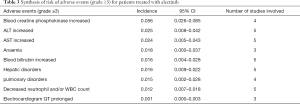
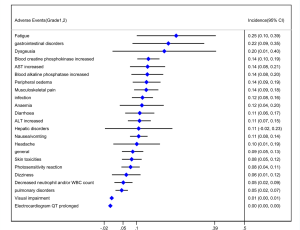
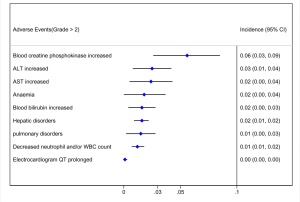
Out of 10 included studies, Ito et al. (20) and Nishio et al. (21) did not provide AE, and AE provided by Novello et al. (22) was discarded because of the absence of grading. A total of 7 studies were used and combined to produce the AEs of alectinib treatment. Due to between-study bias in the description and grading, the AEs were redefined and regraded. In the end, the pooled incidence was obtained as follows: 28 AEs grade 1-grade 2 and 9 AEs ≥ grade 3. The details, including the number of included studies for each of the combined AEs, are shown in Tables 2 and 3. The data revealed that grade 1–grade 2 had the following five side effects with the highest incidence: fatigue 24.7%, gastrointestinal disorders increased 21.8%, dysgeusia 20.3%, AST increased 14.1%, peripheral edema 13.7%, see Figure 9; the grade ≥3 had the following four side effects with the highest incidence: blood creatine phosphokinase increased 5.6%, ALT increased 2.5%, AST increased 2.4% and anemia 1.8%. The forest plots are shown in Figures 10 and 11.
Full table
Full table
Risk of bias across studies
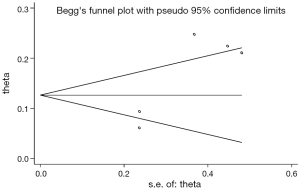
The comparison that was chosen for Egger’s test was the cumulative incidence of CNS progression of alectinib at the 12th month. That yielded P=0.042, suggesting publication bias, so we applied the trim-and-fill method. No trimming or filling was performed, and the data were unchanged. This result indicated that the bias was not very large and did not need to be corrected (Figure 12).
Discussion
A total of 10 studies enrolling 2,377 patients were included in this meta-study. This study revealed that the alectinib group was associated with significantly longer PFS than the crizotinib group; the pooled cumulative incidence of CNS progression was 10% and 16% at the 6th and 12th months after alectinib therapy, respectively; grade 1–grade 2 patients treated with alectinib were associated with the highest incidence of the following side effects: increased blood creatinine, fatigue, musculoskeletal pain, gastrointestinal disorders, and dysgeusia. Grade 3 patients treated with alectinib were associated with the highest incidence of the following side effects: increased blood creatine phosphokinase, increased ALT, increased AST and anemia.
In 2007, ALK gene rearrangement was discovered in NSCLC for the first time in solid tumors (23). Chromosome rearrangement of anaplastic lymphoma kinase (ALK) defines a unique molecular subset that occurs in 3% to 7% of patients with NSCLC. The rearrangement of ALK on chromosome 2 results in the expression of oncogenic ALK fusion and increases the activity of tyrosine kinase in the ALK receptor. ALK fusion proteins, such as echinoderm microtubule-associated protein-like 4 (EML4), can promote tumor cell proliferation and survival by abnormal activation of intracellular signals (23,24). The EML4-ALK protein contains the amino-terminal half of EML4 and the intracellular catalytic domain of ALK. This region of EML4 results in constitutive dimerization of the kinase domain of ALK, leading to aberrant activation of downstream signaling, such as Akt and STAT3 signaling, and extracellular signal regulated kinase 1 and 2 (ERK1/2) (23).
Alectinib was designed as an effective and selective ALK inhibitor to bypass crizotinib resistance (25). Alectinib inhibits ALK autophosphorylation as well as the phosphorylation of signal transducer and activator of transcription 3 (STAT3). ALK-resistant mutations appear to be the main mechanism of resistance for second-generation ALK inhibitors (26). In vitro, alectinib can effectively treat several crizotinib-resistant mutations in ALK, including L1196M, F1174L, R1275Q and C1156Y. Among them, L1196M is known as a gatekeeper mutation in crizotinib drug-resistant mutants. Alectinib can selectively inhibit the growth of L1196M-driven tumors (26). It has shown activity against ALK+ cells with the gatekeeper ALK L1196M mutation, which confers resistance to crizotinib (27). In addition to bypassing crizotinib-resistant mutants in vitro, mouse models of human xenografts with crizotinib-resistant ALK mutations are also sensitive to alectinib; thus, tumor growth is inhibited (27). In addition, cKIT gene amplification, concomitant EGFR-activating mutation (L858R) or KRAS mutation, and activation of insulin-like growth factor 1 receptor were identified as other crizotinib-resistance mechanisms in vitro and in vivo (28,29). Alectinib, which is structurally distinct from crizotinib, may be effective in re-inducing remission when cancers are still dependent on ALK (30). Although many clinical trials have shown impressive results, the exact efficacy of alectinib is still unclear due to its short history, insufficient number of users and short observation time. To confirm the effect of long-term treatment, a large number of specific studies are required. Therefore, we performed this meta-analysis and revealed that the alectinib group had an obviously longer PFS than the crizotinib group, and as a second-generation ALK inhibitor, alectinib showed higher systemic efficacy.
In advanced NSCLC patients with ALK-positive, the CNS is a major and very common metastatic site, with up to 30% of patients having brain metastases at the time of diagnosis. Many patients exhibit metastases within 1 year of first-line crizotinib treatment, usually in the CNS. Crizotinib is a first-in-class ALK/ROS1/MET inhibitor that was initially approved by the US FDA in 2011 for the treatment of advanced NSCLC with ALK-positive. Crizotinib treatment is prone to CNS progression, which is caused by poor accumulation of drugs in the CNS. Crizotinib is a substrate for p-glycoprotein, which is a key efflux transporter located at the blood-brain barrier. Crizotinib can be cleared away from the CNS as a result of p-glycoprotein-mediated efflux through the blood-brain barrier, so it cannot effectively block brain metastasis (31). However, alectinib is not the substrate of p-glycoprotein; it is the major regulator of osmosis in the CNS, and it can effectively cross the blood-brain barrier. Alectinib showed similar free concentrations in plasma and cerebrospinal fluid (CSF) in small mouse brain transfer xenograft models, showing the excellent ability of alectinib to penetrate CNS tissue (32). In the NP28761 study, alectinib showed an overall response rate (ORR) of 75% (12/16) in crizotinib-resistant NSCLC patients with measurable CNS lesions (15). The NP28673 study reported a CNS ORR of 57% in similar crizotinib-resistant patients (17). In our meta-analysis, we combined 5 clinical trials, including two RCTs, and found that the pooled cumulative incidence of CNS progression was 10% and 16% at the 6th and 12th months of alectinib therapy, respectively. This demonstrates the superior CNS protective activity of alectinib, which prevents progression of CNS metastasis in patients with baseline CNS metastases, and it prevents the development of new CNS lesions in patients without baseline CNS metastasis. These studies suggest that alectinib is the choice in patients with NSCLC who are resistant to crizotinib, and alectinib improves systemic and CNS metastases control, thereby improving overall survival. However, this needs to be confirmed in a future analysis of survival event data over a longer time period.
The reduction of systemic AEs is a key component in the age of personalized medicine and targeted therapy. Of all the ALK inhibitors, alectinib is considered to be well tolerated, and few patients refuse treatment because of AEs (33). Alectinib is a highly specific ALK inhibitor, and ALK is only expressed at low levels in normal adult tissues; therefore, alectinib has very limited effects on the systemic function of the body because of its inhibition of ALK. With fewer side effects, doctors can minimize the impact on patient quality of life by giving low doses of alectinib when possible without compromising its effectiveness. Although there are many relevant studies, there are still many uncertainties about the side effects of alectinib. Therefore, our meta-analysis combines 7 studies to analyze the side effects of alectinib therapy and obtain more accurate data, which in turn helps clinicians use alectinib more rationally.
The most common side effects of crizotinib were gastrointestinal AEs, and severe gastrointestinal AEs significantly decreased patient compliance. In the 255 patients receiving treatment with crizotinib, regardless of the class of patients, gastrointestinal AEs were nausea (53%), diarrhea (43%), vomiting (40%), and constipation (27%). These severe gastrointestinal AEs significantly reduced patient compliance. Alectinib has much milder gastrointestinal AEs. In a study of 253 ALK-rearranged NSCLC patients treated with alectinib at 600 mg twice daily, for all classes of patients, the incidence of constipation, diarrhea, nausea and vomiting was 34%, 18%, 16%, and 12%, respectively. This study combined 7 studies and revealed that in grade 1 and grade 2 patients, the incidence of gastrointestinal disorders was 21.8%, which was significantly lower than that of crizotinib, and lower than that in ≥ grade 3 patients, and no significant gastrointestinal disorders were found. Compared with crizotinib, alectinib has better gastrointestinal tolerance, which may lead to better patient compliance and less need for intervention with anti-nausea or vomiting drugs. Thus, when choosing an ALK inhibitor, one of the advantages of choosing alectinib is that fewer anti-vomiting drugs are needed.
Although the incidence of gastrointestinal AEs with alectinib is lower, higher rates of hepatic or musculoskeletal AEs have occurred. Among all AEs observed with alectinib, the most prominent was hepatotoxicity, with ALT increased incidence 2.5% and AST increased incidence 2.4%. Most hepatotoxicity occurs in the first 2 months of alectinib treatment, so it is necessary to closely monitor liver function early in the treatment process, especially for patients with preexisting hepatic impairment due to liver metastases. Myalgia and elevation of CPK (creatine phosphokinase) are also AEs that require close attention. We found that in grade 1 and grade 2 patients, creatine phosphokinase increased reached 25.3%, and musculoskeletal pain reached 22.4%; in grade ≥3 patients, blood creatine phosphokinase increased reached 5.6%. Among all ALK inhibitors, alectinib has unique side effects that deserve our attention. Some studies have reported that sinus bradycardia is a side effect unique to alectinib, but our meta-analysis did not find significant sinus bradycardia. This may be related to the absence of symptoms in most patients. For symptomatic patients, careful medical treatment should be performed first to eliminate the drugs that may cause sinus bradycardia and then to adjust the dose of alectinib for specific patients. Taken together, the safety profile of alectinib and its clinical efficacy have proven that it is a meaningful treatment option for patients with ALK-rearranged NSCLC. Despite such satisfactory effects, there is no direct evidence that alectinib can replace crizotinib.
Certain limitations of this study must be considered. First, of the 11 clinical trials included in this study, only 4 were RCTs, and the other 7 were retrospective or prospective single-arm cohort trials. Second, the recommended dose of alectinib used in clinical trials in Japan was 300 mg twice daily, while outside of Japan, it was 600 mg twice daily. Nevertheless, the outcomes of clinical trials in Japan are consistent with those in other countries, so this conclusion has certain value and significance.
Conclusions
Taken together, the results indicate that alectinib significantly prolongs PFS, and it better controls CNS metastases than crizotinib; however, there is insufficient evidence that alectinib could completely replace crizotinib. Although alectinib has a smaller gastrointestinal response than crizotinib, it still is associated with prominent liver damage and myalgia, which is worthy of attention. This systematic review and meta-analysis can provide some references for the clinical use of alectinib.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81570993) and Basic Research Fund of Central Public Welfare Scientific Institute (grant number 2016ZX310181-6).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-643). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Sullivan I, Planchard D. Treatment modalities for advanced ALK-rearranged non-small-cell lung cancer. Future Oncol 2016;12:945-61. [Crossref] [PubMed]
- Khan M, Lin J, Liao G, et al. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front Oncol 2019;8:557. [Crossref] [PubMed]
- Sgambato A, Casaluce F, Maione P, et al. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer Ther 2018;18:71-80. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem 2012;20:1271-80. [Crossref] [PubMed]
- Karachaliou N, Fernandez Bruno M, Bracht JWP, et al. Profile of alectinib for the treatment of ALK-positive non-small cell lung cancer (NSCLC): patient selection and perspectives. Onco Targets Ther 2019;12:4567-75. [Crossref] [PubMed]
- Tsuji T, Ozasa H, Aoki W, et al. Alectinib Resistance in ALK-Rearranged Lung Cancer by Dual Salvage Signaling in a Clinically Paired Resistance Model. Mol Cancer Res 2019;17:212-24. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK -positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Tamura T, Kiura K, Seto T, et al. Three-Year Follow-Up of an Alectinib Phase I/II Study in ALK-Positive Non-Small-Cell Lung Cancer: AF-001JP. J Clin Oncol 2017;35:1515-21. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. The Lancet Oncology 2016;17:234-42. [Crossref] [PubMed]
- Masuda N, Ohe Y, Gemma A, et al. Safety and effectiveness of alectinib in a real-world surveillance study in patients with ALK-positive non-small-cell lung cancer in Japan. Cancer Sci 2019;110:1401-7. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Ito K, Hataji O, Kobayashi H, et al. Sequential Therapy with Crizotinib and Alectinib in ALK-Rearranged Non-Small Cell Lung Cancer-A Multicenter Retrospective Study. J Thorac Oncol 2017;12:390-6. [Crossref] [PubMed]
- Nishio M, Nakagawa K, Mitsudomi T, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2018;121:37-40. [Crossref] [PubMed]
- Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Muller IB, de Langen AJ, Giovannetti E, et al. Anaplastic lymphoma kinase inhibition in metastatic non-small cell lung cancer: clinical impact of alectinib. Onco Targets Ther 2017;10:4535-41. [Crossref] [PubMed]
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679-90. [Crossref] [PubMed]
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215-21. [Crossref] [PubMed]
- Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol 2013;31:3987-96. [Crossref] [PubMed]
- Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res 2015;21:2227-35. [Crossref] [PubMed]
- Tomasini P, Egea J, Souquet-Bressand M, et al. Alectinib in the treatment of ALK-positive metastatic non-small cell lung cancer: clinical trial evidence and experience with a focus on brain metastases. Ther Adv Respir Dis 2019;13:1753466619831906. [Crossref] [PubMed]
- Tang SC, Nguyen LN, Sparidans RW, et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 2014;134:1484-94. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]
- Zhu V, Ou SH. Safety of alectinib for the treatment of metastatic ALK-rearranged non-small cell lung cancer. Expert Opin Drug Saf 2017;16:509-14. [Crossref] [PubMed]