Platelet maximum aggregation rate serves as a marker in diagnosis and prognosis in patients with sepsis
Introduction
The detection of platelet aggregation began in the 1960s, using plasma nephelometry (1). The assessment of platelet aggregation rate is now widely used in the management of patients with a range of conditions, as well as in the monitoring of antiplatelet drug use and the in vitro diagnostic testing of platelet function (2). New methods of assessing platelet aggregation have been developed, such as the whole blood continuous counting platelet function analyzer PL-12, but few studies have assessed platelet aggregation in patients with sepsis.
Sepsis is caused by dysregulation of the host’s response to infection and can lead to life-threatening organ dysfunction. Septic shock refers to lactic acid levels ≥2 mmol/L combined with insufficient blood volume. Organ dysfunction is defined as two or more organ dysfunctions according to the Sequential Organ Failure Assessment (SOFA) scoring system (3). Sepsis is a worldwide problem and is associated with a mortality rate of approximately 30−50% (4). Physiological changes and a reduction in blood coagulation factors and platelets leads to the clotting dysfunction characteristic of sepsis. Subsequent complications include disseminated intravascular coagulation (DIC) and multiple organ failure, which in turn affects the prognosis of patients with sepsis (5). As the progression of sepsis may involve changes in platelet aggregation, an early understanding of platelet aggregation, allowing early intervention in patients with sepsis, may be critical to control patient’s condition.
Methods
Patients
The study population comprised patients with sepsis (defined by the 2016 international diagnostic criteria for sepsis 3.0) (2) treated in the intensive care unit (ICU) of the Xiangya Third Hospital between Sep 2017 and Apr 2018. Patients were excluded if they had blood system diseases, immune diseases, malignant tumors, or organ transplantation; patients were also excluded if they were pregnant, were undergoing dialysis, or had received anticoagulation agents, antiplatelet aggregation drugs, or any medication that may affect platelet function in the past 2 weeks. Blood samples were taken within 24 hours, and procalcitonin (PCT), C-reactive protein (CRP), routine blood tests, liver and kidney function, blood culture, and coagulation function indicators were determined. SOFA scores were calculated over the first 24 hours after the severe sepsis criteria had been met (6). All patients were followed up for 30 days to determine the survival.
A control group of healthy volunteers was also included, which comprised patients from the physical examination center of the hospital who visited in the same period. Subjects were excluded if they had hypertension, coronary heart disease, diabetes, hepatitis, thrombosis, serious infection, or other serious diseases of the heart, brain, lung, liver, and kidney; they were also excluded if they were pregnant or had received anticoagulation or antiplatelet aggregation drugs in the previous 2 weeks. Blood samples were collected on the same day to detect platelet aggregation.
The study was conducted following the appropriate standards of medical ethics, and the study design was approved by the institutional ethics committee of Xiangya Third Hospital, Central South University (2018-S114). Informed consent was obtained from patients or family members.
Platelet aggregation function test
Venous blood was collected in 3 mL sodium citrate anticoagulation vacuum plastic tubes and processed within 3 hours in accordance with the guidelines on the Evaluation of Precision of Quantitative Measurement Procedures produced by the Clinical and Laboratory Standards Institute (EP05-A3) (7). Whole blood samples were pipetted into the platelet function analyzer along with one of four inducers [peanut four arachidonic acid (AA), adenosine diphosphate (ADP), epinephrine (EPI), and collagen (COL)] or saline. The PL-12 platelet analyzer, related reagents, and inducers were supplied by Nanjing Shenzhou Yingnuohua Medical Technology Co., Ltd.
Assessment of coagulation function, PTC, CRP, and biochemical function
After the diagnosis of sepsis, blood samples (10 mL) were collected from a peripheral vein or central venous catheter in all subjects. Samples were evaluated using the Mindray CAL 800. Coagulation function was tested using the automatic coagulation analyzer CS-5100. PCT and CRP were tested using the mini IDAs immunofluorescence quantitative analyzer using the chemiluminescence method. Biochemical function was assessed using the Hitachi 7600-010 automatic biochemical analyzer.
Statistical evaluation
Data were analyzed and processed using SPSS 22 statistical software. For data with a normal distribution, mean ± standard deviation is used for descriptive analysis. Median values and quartiles are used for nonparametric data. Descriptive analysis was performed by the number ± quartile interval; differences between the groups (experimental and control, survivor and non-survivor) were compared by t-test, and the paired samples were compared. Kaplan-Meier analysis was used to assess the relationship between 28-day survival and the results of impedance aggregation assays. For the classification data, descriptive analysis was performed using the frequency and composition ratio; the comparison between the two samples was performed by chi-square test. Impedance aggregation assays, diagnostic and prognostic values for conventional biomarkers, and coagulation indicators were also determined by multivariate analysis using logistic regression. Correlation was performed using linear regression analysis. Using the receiver operating characteristic curve and the corresponding region under the curve, the asymptotic saliency and the 95% asymptotic confidence interval compared the impedance aggregation measurement results with the conventional biomarkers. All P values represent a two-sided probability with a test level of α =0.05.
Results
General data
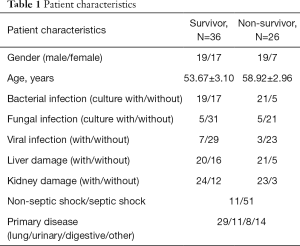
Sixty-two patients with sepsis were treated during the study period and met the inclusion requirements; 38 patients were males and 24 were females, with an average age of 55.9±2.2 years. During the follow-up, 26 patients died and 36 survived. The characteristics of the patient cohort are shown in Table 1. The control group comprised 37 subjects, including 21 males and 16 females, with an average age of 49.1±1.6 years.
Full table
Platelet aggregation rate as a biomarker for diagnosis of sepsis
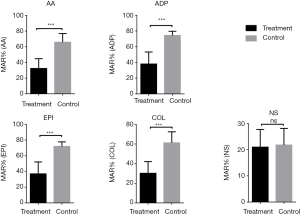
Decreased platelet aggregation was seen in patients with sepsis with all four activators (Figure 1); detailed values are shown in Table 2. As shown in Figure 2, the maximum aggregation rate (MAR) of platelets in the ADP group and EPI group was higher than that in the AA and COL groups.

Full table
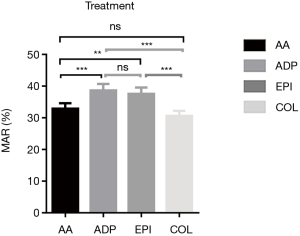
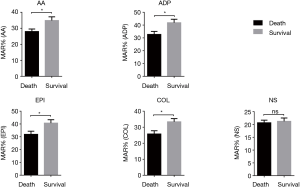
Figure 3 shows the results obtained in the survivor and non-survivor groups, demonstrating a lower MAR in non-survivors. Table 3 summarizes the results of the agglutination assays, conventional sepsis markers, SOFA scores, and conventional coagulation variables in the two groups.
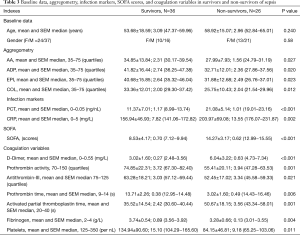
Full table
To further compare the impedance aggregation assay with conventional biomarkers, a receiver operating characteristic curve was generated from the variables (Figure 4). The area under the curve as an indicator of the reliability of the measurements obtained from these curves, and the significance level and confidence intervals, are shown in Table 4. The results show that the SOFA score was the most sensitive indicator for diagnosis of sepsis, and collagen used as an activator was a better biomarker for sepsis. PCT is more sensitive than CRP in the index of infection. Among the coagulation variables, D-dimer was seen to be the better biomarker of sepsis. In the logistic regression analysis, PCT and D-dimer were seen to be independent risk factors (Table 5).
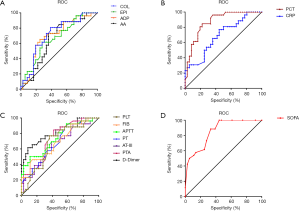
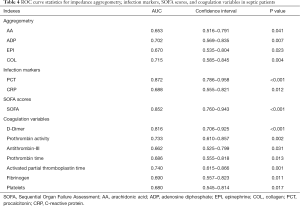
Full table

Full table
Comparison of the maximum platelet aggregation rate and platelet count
The results of the impedance aggregation assay may be affected by the platelet count. To exclude the influence of platelet count on the impedance aggregation assay, the two variables were compared and a correlation study was performed. The platelet count was 163/nL (range, 154−172/nL) in healthy subjects and 114/nL (93−134/nL) in patients with sepsis (Table 2). Comparison of impedance aggregation assay results and platelet counts showed only a low correlation with each of the four activators in sepsis patients (R2: range, 0.003−0.034) and the control group (R2: range, 0.071−0.197; Figure 5).
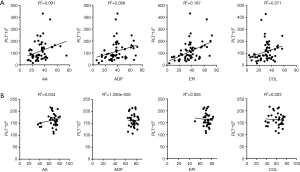
Biomarkers for diagnosis and prognosis of sepsis
Among the coagulation markers, D-dimer, prothrombin activity, antithrombin-III (AT-III), prothrombin time, activated partial thromboplastin time, and fibrinogen showed predictive significance for diagnosis and prognosis of sepsis. The SOFA score not only diagnosed septic disease, but also determined the prognosis of sepsis. PCT and CRP in the infection index is of predictive significance for diagnosis and prognosis of sepsis, among which D-dimer, SOFA score, and PCT were excellent markers for diagnosis of sepsis (Tables 3,4).
Discussion
The whole blood platelet function analyzer PL-12 is a new platelet aggregation rate assay that relies on measuring changes in impedance caused by platelet aggregation on electrodes in whole blood samples (8) without the need for centrifugation and purification. Each sample can produce results within 6 minutes, and dual measurements in each vial allow for automatic quality control. The cost per measurement is also relatively low. The results of the study presented here demonstrate that the MAR of platelets is a good predictor of diagnosis of sepsis and the outcome of patients with severe sepsis. Notably, among the four activators, collagen as an activator was seen to be a better biomarker for diagnosis of sepsis and sepsis survival.
In patients with sepsis, inhibition of platelet function in experimental sepsis has shown promising results (9). Platelets promote inflammation in the early stages of infection, but subsequently inhibit cytokine release when the inflammatory response is increased (10). Decreased aggregation in patients with severe sepsis and loss of platelet function in patients with multiple organ dysfunction syndrome (11), especially poor prognosis, suggests that platelet inhibition may be at a calculated risk (12). The maximum platelet aggregation and secretion in the first wave of aggregation is significantly reduced, and the first wave of aggregation is usually associated with the activity of several platelet mitochondrial respiratory chain enzymes. Studies have shown that platelet mitochondrial respiratory chain enzymes are inhibited during infection, mainly through inflammatory mediators (13). Animal studies have shown that lipopolysaccharide (LPS) enhances reactive oxygen species (ROS) in a mouse model of LPS treatment. Production of ROS, which activates the production of polyethylene glycol superoxide dismutase (peg-sod), peg-catalase, and NADPH oxidase inhibitor (DPI), thereby enhances the inhibition of platelet aggregation by LPS, which is related to LPS dose (14). Therefore, the more severe the infection, the more the platelet mitochondrial respiratory chain enzymes are inhibited and the lower the platelet aggregation rate. This is also consistent with the results of the current study. Once activated, platelets release TxA2 and ADP molecules, which amplify platelet activation exponentially through autocrine or paracrine pathways. In sepsis-related dysfunction, the cyclooxygenase pathway may change to reduce the response to AA, thereby reducing the affinity for fibrinogen (15), collagen receptors, and coagulation in sepsis. The agonist of the enzyme receptor (PAR-1) is affected by the defect that leads to the level of intracellular transduction pathway; thus collagen and AA-induced MAR will decrease (16). This is consistent with the results of the current study, which show that COL and AA-induced platelet aggregation is consistent with ADP and EPI-induced platelet aggregation.
Thrombocytopenia in patients with sepsis is closely associated with poor outcomes and higher mortality (17). This fact was also confirmed in the single factor study of sepsis in the current study, in which the platelet count in the non-survivor group was seen to be significantly lower than that in surviving patients. Sepsis is often associated with coagulopathy, and it is estimated that >80% of patients with sepsis have varying degrees of clinical or subclinical coagulopathy, which also increases the mortality risk (18). In addition, AT-III is an important antithrombin in the body, which can consume a large amount of thrombin and inhibit the endotoxin-induced inflammatory reaction. Studies have shown that patients with sepsis will have a decrease in AT-III levels, which can be used as a prognostic indicator (19). Approximately 35% of cases of severe sepsis are accompanied by DIC (20). DIC can cause a series of reactions, such as platelet depletion, coagulation system and anticoagulant system activation, complement activation, and neutrophil activation (21). In patients with DIC, the coagulation cascade and fibrinolytic system are simultaneously activated. As DIC develops, prothrombotic and antithrombotic factors, as well as fibrinolytic and antifibrinolytic factors, are consumed resulting in a hemorrhagic or thrombotic state. This positive feedback loop is promoted by the immune system and the coagulation pathway (22). Sepsis-associated coagulopathy (SAC) can promote DIC and multiple organ failure (23). Platelet activation is mainly caused by bacteria, not sepsis-related thrombocytopenia (24). Platelet activation markers have been seen to be significantly elevated in SAC patients compared with healthy individuals. This increase is independent of platelet counts, and markers of platelet activation in SAC are primarily regulated by the number of circulating platelets and may be independent of factors that contribute to their endogenous consumption (25). As shown in the current study, the relationship between platelet count and platelet aggregation was not significant.
Study on surviving and non-surviving sepsis patients has demonstrated that the presence of abnormal coagulation at admission is independently association with increased short- and medium-term mortality (26). Studies have also shown that D-dimer and FDP levels can distinguish between ICU patients with and without sepsis and are a statistically significant, time-dependent predictor of survival (27). These data are also consistent with the results of the current study.
Neutrophils phagocytose and eliminate microbes through oxidative and non-oxidative mechanisms, which plays an important role in the host’s inflammatory response to invading pathogens (28). Neutrophils are therefore thought to be the main participants in the host’s immune response to sepsis (29), as they contain highly toxic proteolytic enzyme granules that can be released to form neutrophil extracellular reticular traps (NETs). DNA constitutes a reticular structure involved in immunity (30). Studies have shown that neutrophils from patients with sepsis show increased production of ROS, which is highly toxic to host tissues (31). NETs also have potential adverse effects on host tissues during sepsis (32); therefore, excessive stimulation of neutrophils is thought to be responsible for multiple organ failure during sepsis (33). It has also been demonstrated that neutrophils are associated with the condition of patients with sepsis. In a mouse model of sepsis, LPS resulted in a rapid and transient increase in systemic cytokine levels and peripheral vascular resistance (34). Cytokines are endogenous polypeptides or glycoproteins that are induced by tumor necrosis factor, and changes in pro-inflammatory cytokines and CRP play an important role in septic shock or multiple organ failure (35). PCT has been suggested as a reliable biomarker for predicting sepsis (36). The concentration of PCT became apparent as time elapsed after LPS injection. And the degree of sepsis is significant over time, and this study also confirmed that PCT, CRP, and other infectious markers play an important role in diagnosis and prognosis of sepsis.
The SOFA score ranges from 0 to 24, with a score of 0–6 being associated with an expected mortality rate <10%; a score of 13–14 is associated with a 50% mortality rate, and a score >15 indicates a mortality rate of 90% (37). Therefore, the higher the score, the greater the risk of death. In the current study, the importance of the SOFA score for diagnosis of sepsis and the predictability of prognosis was also confirmed.
Conclusions
The results of the current study suggest that early detection of platelet aggregation on the first day of ICU admission may serve as a new and reliable biomarker for sepsis. It can also be used to assess changes in the patient’s condition and to guide treatment. A decrease in platelet aggregation rate caused by infection is not necessarily dependent on platelet count. The whole blood platelet function analyzer PL-12 is a new type of platelet aggregation detection method that has the potential to facilitate assessment in clinical practice.
Acknowledgments
Funding: This work was supported by grants from General Program of Natural Science Foundation of Hunan Province of China (Grant No. 2018JJ2603).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study design was approved by the institutional ethics committee of Xiangya Third Hospital, Central South University (2018-S114). Informed consent was obtained from patients or family members.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Napolitano LM. Sepsis 2018: Definitions and Guideline Changes. Surg Infect (Larchmt) 2018;19:117-25. [Crossref] [PubMed]
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762-74. [Crossref] [PubMed]
- Jozwiak M, Hamzaoui O, Monnet X, et al. Fluid resuscitation during early sepsis: a need for individualization. Minerva Anestesiol 2018;84:987-92. [Crossref] [PubMed]
- Halbgebauer R, Schmidt CQ, Karsten CM, et al. Janus face of complement-driven neutrophil activation during sepsis. Semin Immunol 2018;37:12-20. [Crossref] [PubMed]
- Innocenti F, Tozzi C, Donnini C, et al. SOFA score in septic patients: incremental prognostic value over age, comorbidities, and parameters of sepsis severity. Intern Emerg Med 2018;13:405-12. [PubMed]
- Wang XW, Niu XG, Li JX, et al. SOFA Score Can Effectively Predict the Incidence of Sepsis and 30-Day Mortality in Liver Transplant Patients: A Retrospective Study. Adv Ther 2019;36:645-51. [Crossref] [PubMed]
- Sahu C, Jain V, Mishra P, et al. Clinical and laboratory standards institute versus European committee for antimicrobial susceptibility testing guidelines for interpretation of carbapenem antimicrobial susceptibility results for Escherichia coli in urinary tract infection (UTI). J Lab Physicians 2018;10:289-93. [Crossref] [PubMed]
- Sharma RK, Voelker DJ, Sharma R, et al. Evolving role of platelet function testing in coronary artery interventions. Vasc Health Risk Manag 2012;8:65-75. [Crossref] [PubMed]
- Winning J, Reichel J, Eisenhut Y, et al. Anti-platelet drugs and outcome in severe infection: clinical impact and underlying mechanisms. Platelets 2009;20:50-7. [Crossref] [PubMed]
- Ware J, Post SR. Platelets: balancing the septic triad. Blood 2014;124:3670-2. [Crossref] [PubMed]
- Lundahl TH, Petersson J, Fagerberg IH, et al. Impaired platelet function correlates with multi-organ dysfunction. A study of patients with sepsis. Platelets 1998;9:223-5. [Crossref] [PubMed]
- Adamzik M, Gorlinger K, Peters J, et al. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit Care 2012;16:R204. [Crossref] [PubMed]
- Protti A, Fortunato F, Artoni A, et al. Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Crit Care 2015;19:39. [Crossref] [PubMed]
- Aydemir H, Piskin N, Akduman D, et al. Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets 2015;26:331-5. [Crossref] [PubMed]
- Lopes-Pires ME, Casarin AL, Pereira-Cunha FG, et al. Lipopolysaccharide treatment reduces rat platelet aggregation independent of intracellular reactive-oxygen species generation. Platelets 2012;23:195-201. [Crossref] [PubMed]
- Lundahl TH, Lindahl TL, Fagerberg IH, et al. Activated platelets and impaired platelet function in intensive care patients analyzed by flow cytometry. Blood Coagul Fibrinolysis 1996;7:218-20. [Crossref] [PubMed]
- Hampton T. Platelets' Role in Adaptive Immunity May Contribute to Sepsis and Shock. JAMA 2018;319:1311-2. [Crossref] [PubMed]
- Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 2018;16:231-41. [Crossref] [PubMed]
- Pannu AK, Saroch A, Sharma N. Danger Triangle of Face and Septic Cavernous Sinus Thrombosis. J Emerg Med 2017;53:137-8. [Crossref] [PubMed]
- Ishikura H, Nishida T, Murai A, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care 2014;18:R19. [Crossref] [PubMed]
- Umemura Y, Yamakawa K, Ogura H, et al. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost 2016;14:518-30. [Crossref] [PubMed]
- Ding R, Wang Z, Lin Y, et al. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis 2018;29:551-8. [Crossref] [PubMed]
- Kapoor S, Opneja A, Nayak L. The role of neutrophils in thrombosis. Thromb Res 2018;170:87-96. [Crossref] [PubMed]
- Samuels JM, Moore HB, Moore EE. Coagulopathy in Severe Sepsis: Interconnectivity of Coagulation and the Immune System. Surg Infect (Larchmt) 2018;19:208-15. [Crossref] [PubMed]
- Conway-Morris A, Wilson J, Shankar-Hari M. Immune Activation in Sepsis. Crit Care Clin 2018;34:29-42. [Crossref] [PubMed]
- Johansson D, Rasmussen M, Inghammar M. Thrombocytopenia in bacteraemia and association with bacterial species. Epidemiol Infect 2018;146:1312-7. [Crossref] [PubMed]
- Davies RS, Abdelhamid M, Vohra RK, et al. The relationship between aortic aneurysm sac thrombus volume on coagulation, fibrinolysis and platelet activity. Thromb Res 2012;130:463-6. [Crossref] [PubMed]
- Innocenti F, Gori AM, Giusti B, et al. Prognostic value of sepsis-induced coagulation abnormalities: an early assessment in the emergency department. Intern Emerg Med 2019;14:459-66. [Crossref] [PubMed]
- Toh JM, Ken-Dror G, Downey C, et al. The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis 2013;24:839-43. [Crossref] [PubMed]
- Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006;6:541-50. [Crossref] [PubMed]
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862-74. [Crossref] [PubMed]
- Ng TH, Chang SH, Wu MH, et al. Shrimp hemocytes release extracellular traps that kill bacteria. Dev Comp Immunol 2013;41:644-51. [Crossref] [PubMed]
- Li RHL, Tablin F. A Comparative Review of Neutrophil Extracellular Traps in Sepsis. Front Vet Sci 2018;5:291. [Crossref] [PubMed]
- Grailer JJ, Kalbitz M, Zetoune FS, et al. Persistent neutrophil dysfunction and suppression of acute lung injury in mice following cecal ligation and puncture sepsis. J Innate Immun 2014;6:695-705. [Crossref] [PubMed]
- Urban C, Zychlinsky A. Netting bacteria in sepsis. Nat Med 2007;13:403-4. [Crossref] [PubMed]
- Liu L, Sun B. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol Life Sci 2019;76:2031-42. [Crossref] [PubMed]
- Yeom E, Kim HM, Park JH, et al. Microfluidic system for monitoring temporal variations of hemorheological properties and platelet adhesion in LPS-injected rats. Sci Rep 2017;7:1801. [Crossref] [PubMed]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 2005;4:854-65. [Crossref] [PubMed]
- Amer HA, Ghareeb H, Lotfy NM, et al. Presepsin a Diagnostic Marker for Sepsis in Intensive Care Unit Patients. Egypt J Immunol 2016;23:109-18. [PubMed]
- Middleton E, Rondina MT. Platelets in infectious disease. Hematology Am Soc Hematol Educ Program 2016;2016:256-61. [Crossref] [PubMed]
- Gaini S, Relster MM, Pedersen C, et al. Prediction of 28-days mortality with sequential organ failure assessment (SOFA), quick SOFA (qSOFA) and systemic inflammatory response syndrome (SIRS) - A retrospective study of medical patients with acute infectious disease. Int J Infect Dis 2019;78:1-7. [Crossref] [PubMed]