Clinical effectiveness of matrine sitz bath in treating perianal infection after chemotherapy for acute leukemia
Introduction
Acute leukemia is a malignant transformation and proliferation of lymphoid progenitor cells. As a highly fatal condition, it accounts for about 3% of malignant tumors, with a mortality rate of 3.4 per 100,000 individuals (1,2). Depending on the blood cells it affects, acute leukemia can be divided into acute myelogenous leukemia (AML) and acute lymphocytic leukemia (ALL). The currently available treatments for acute leukemia include chemotherapy, immunotherapy, and targeted therapy, with chemotherapy being the most commonly used treatment. However, chemotherapy weakens the immune systems and causes bone marrow suppression (3,4), leading to complications such as bleeding and infection, among which perianal infection is one of the most common infectious complications. Perianal infection was caused by many factors, more than 90% of which originated from the anal gland infection between the sphincter. The incidence of perianal infection in Chinese patients with acute leukemia receiving chemotherapy was 13.9% (5), and perianal infection is also one of the main causes of death in patients with acute leukemia (6,7). At present, fumigation, physiotherapy and wet application are the main methods to treat perianal infection in leukemia patients. However, these treatment methods have the disadvantages of cumbersome operation, high cost and poor acceptance of patients. Patients with perianal infection usually have clinical symptoms such as perianal mass, redness/swelling, heat, and pain, which affect the efficacy of chemotherapy and lead to poor physical and mental health. Therefore, active prevention and treatment of perianal infection are particularly important for improving the physical and mental health of acute leukemia patients and increasing their survival rate. Traditional Chinese medicine sitting bath treatment of acute leukemia patients with perianal infection has a good effect, but it also has many disadvantages: the production method is complex, sitting bath liquid must be modulated, and the modulation method is not easy to grasp. This method not only brings great inconvenience to the patients, but also increases the workload of the medical staff. Matrine sitting bath (MSB) preparation method is simple. The purpose of our current study was to investigate the effectiveness of MSB in treating perianal infection after chemotherapy for acute leukemia in terms of improving clinical symptoms and alleviating inflammatory reactions. We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/apm-20-912).
Methods
Subjects
A total of 216 patients suffering from perianal infection during chemotherapy for acute leukemia in our center from June 2015 to December 2019 were enrolled in this study. Using the random number table method, they were equally randomized into two groups: the MSB group and the control group. The general data were matched between these two groups (all P>0.05) (Table 1).

Full table
The inclusion criteria were the following: (I) the patients met the diagnostic criteria for acute leukemia in the classification of myeloid tumors revised by the World Health Organization in 2008 (8); (II) the perianal infection met the diagnostic criteria (9) of swelling, redness, itching, tenderness during defecation, local pain in the perianal area, and a body temperature of >38.5 °C; (III) the patient’s age was 18 to 65 years, with complete medical histories and clinical data; (IV) patients had received at least two cycles of chemotherapy for acute leukemia; and (V) all patients signed the informed consent.
The exclusion criteria included the presence of (I) other systemic infections; (II) the use of anti-infective drugs within 1 week before enrollment; (III) allergic conditions; (IV) mental disorders; or (V) other accompanying anorectal diseases.
This study was approved by the ethics committee of our hospital [approval document number: (2015)KY037] and was conducted in accordance with the Declaration of Helsinki.
Treatment
During the treatment of the patients, the full-time deputy chief nurse gave technical guidance.
Control group
Patients in the control group were treated with a conventional potassium permanganate sitz bath, which was started 1 day before the patient received chemotherapy. An adequate amount of 1:5,000 potassium permanganate solution was added into the bathtub, and the bathtub temperature was adjusted to 40 °C. The perianal skin was routinely disinfected before the sitz bath. After the disinfection was completed, the patient was instructed to take off his/her clothes and sit in the bathtub. The patient’s buttocks were completely soaked in the potassium permanganate solution. Each sitz bath lasted 20 minutes, and the treatment was performed 3 times a day (morning, noon, and night).
MSB group
Patients in the MSB group received MSB, which was also started 1 day before the patient received chemotherapy. An adequate amount of matrine solution (0.3 g/L) was added into the tub for treatment. The treatment method, duration, and frequency were the same as in the control group.
Patients in both groups were treated for 14 days. The clinicians adjusted the duration of the sitz bath according to the patient’s perianal conditions during the treatment.
Response evaluation criteria
The clinical efficacies of the treatments were evaluated in accordance with the Criteria of Diagnosis and Therapeutic Effect of Diseases and Syndromes in Traditional Chinese Medicine (ZY/T001.1-94) (10). There were four treatment response levels: cured, markedly improved, improved, and no response. The response levels definitions follow below. Cured: the clinical symptoms, anal pain, and perianal mass have basically disappeared; markedly improved: the clinical symptoms, anal pain, and perianal mass have remarkably improved; improved: the clinical symptoms, anal pain, and perianal mass have somewhat improved; no response: the clinical symptoms, anal pain, and perianal mass have not improved or have worsened.
Main measures
Scores of symptoms and signs
Anal pain, systemic symptoms, mass size, and mass texture were recorded in all patients before and after treatment, and each item was scored. Anal pain was rated on an intensity scale of 0= none, 2= mild, 4= moderate, or 6= severe. Systemic symptoms, mass size, and mass texture were rated on an intensity scale of 0= none, 1= mild, 2= moderate, or 3= severe. A higher score indicated a more serious condition.
Detection of inflammatory biomarkers
A total of 5 mL of fasting peripheral blood was drawn from the patients before and after treatment. The serum levels of tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), and prostaglandin E2 (PGE2) were detected according to the instructions of ELISA kits (Shanghai Yuan Ye Biotechnology Co., Ltd., Shanghai, China).The serum levels of high-sensitivity C-reactive protein (hs-CRP) was detected by turbidimetric scattering method with the Dade Behring Lmmage 800 special protein analyzer. The serum levels of Erythrocyte sedimentation rate (ESR) was detected by free sedimentation method with Italian Vital dc-10064 automatic blood sedimentation instrument.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software package. The measurement data are described as mean ± standard deviation and were compared by using the t-test for two independent samples. The count data are presented with rates and percentages and were compared using chi-squared test and rank-sum test. A P value of less than 0.05 was considered statistically significant.
Results
Clinical efficacies in the two groups
The clinical efficacy was significantly higher in the MSB group than in the control group (P<0.05) (Table 2).

Full table
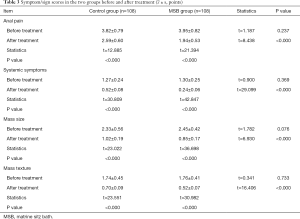
Symptom/sign scores in the two groups before and after treatment
Before treatment, the scores of anal pain, systemic symptoms, mass size, and mass texture showed no significant difference between the MSB group and control group (all P>0.05). After the treatment, these scores significantly decreased in both groups (all P<0.05), and they were significantly lower in the MSB group than in control group (all P<0.05) (Table 3).
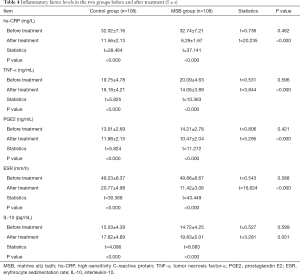
Inflammatory factor levels before and after treatment in the two groups
Before treatment, there was no statistical difference in serum hs-CRP, TNF-α, IL-10, ESR, and PEG2 levels between these two groups (all P>0.05). After treatment, the serum levels of hs-CRP, TNF-α, ESR, and PGE2 in these two groups significantly dropped (P<0.05), and they were significantly lower in the MSB group than in the control group (P<0.05); IL-10 level significantly rose in both groups (P<0.05), and it was significantly higher in the MSB group than in the control group (P<0.05) (Table 4).
Discussion
Perianal infection is one of the most common complications in patients receiving chemotherapy for acute leukemia. Clinically, it often manifests as perianal masses, redness/swelling, heat, and pain. In severe cases, it may result in defecation and urination disorders. The incidence of perianal infection is high in patients with acute leukemia receiving chemotherapy. Typically, it occurs during the myelosuppression period shortly after chemotherapy (11,12), which can cause serious damage to patients’ quality of life and physical and mental health. The traditional treatments for perianal infection are often associated with significant pain, long wound-healing time, and unavoidable complications such as perianal abscess. Matrine is a tetracyclic quinolizine alkaloid derived from the herb Radix Sophorae flavescentis and has anti-inflammatory, anti-tumor, immune-regulating, antipyretic, analgesic, and nerve-stabilizing properties (13-17). As shown in our current study, MSB group had significantly better clinical efficacy than that of the control group, suggesting MSB has good clinical effectiveness in treating perianal infection in acute leukemia patients receiving chemotherapy as it can effectively improve the clinical symptoms and signs in these patients.
Perianal infections are mainly caused by bacterial infections and the common pathogenic bacteria include Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus. Patients with perianal infections can experience both local problems such as perianal skin redness, swelling, and pain, and systemic symptoms including high fever and fatigue. We also compared the changes in symptoms and signs before and after treatment in the two groups. The results suggested that the anal pain, systemic symptoms, mass size, and mass texture were improved after treatment in both the MSB group and control group, and the improvements were more prominent in the MSB group than in the control group. This suggests that MSB can effectively alleviate anal pain and systemic symptoms and improve the size and texture of local masses. Chuang et al. (18,19) reported that matrine could kill a variety of pathogenic bacteria such as Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, possibly by suppressing the biofilms of these pathogens (20). Therefore, matrine may inhibit the infections of pathogenic bacteria and contribute to alleviating anal pain and systemic symptoms, shrinking local masses, and lowering the symptom and sign scores in patients with acute leukemia receiving chemotherapy.
Perianal infection is an inflammatory disease in which inflammatory factors play various key roles. hs-CRP is an acute-phase reactant produced and secreted by the liver. It can participate in the inflammatory response by activating complements, binding to monocyte receptors, and secreting lymphokines. Its level in the human body increases with the severity of infection and thus is clinically valuable for detecting concurrent infections in patients with acute leukemia (21). TNF-α is a major pro-inflammatory cytokine involved in immune response and inflammatory events. Its level markedly increases under pathological conditions (22,23).
PGE 2 is an inflammation mediator that is widely present in human body. It participates in intestinal inflammation and is associated with the severity of certain diseases (24,25). ESR is less specific in the diagnosis of infections but is highly sensitive in reflecting the occurrence and development of inflammation (26). The ESR level markedly increases in cases of infections (27). IL-10 is an anti-inflammatory cytokine. It can reduce inflammatory response by inhibiting the synthesis and release of various pro-inflammatory factors in the body (28). In our current study, the inflammatory response was significantly improved in both the MSB group and control group after treatment, and the improvement was more prominent in the MSB group, suggesting that MSB can effectively adjust the serum levels of hs-CRP, TNF-α, IL-10, ESR, and PEG2 and alleviate inflammatory response in patients with perianal infection after chemotherapy for acute leukemia.
Conclusions
MSB is highly effective in treating perianal infection in patients with acute leukemia receiving chemotherapy as it can effectively improve the symptoms and signs of these patients, decrease serum levels of proinflammatory factors (including hs-CRP, TNF-α, ESR, and PEG2), increase the levels of anti-inflammatory factor levels (e.g., IL-10), and alleviate inflammation.
Acknowledgments
Funding: This work was supported by the Translational Medicine Specialty of Wuxi Municipal Health Committee (ZM006). We thank this fund and the Affiliated Hospital of Jiangnan University for providing experimental equipment support for the study.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-912
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-912
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at available at http://dx.doi.org/10.21037/apm-20-912). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in compliance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University [(2015)KY037]. All subjects agreed and signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Papadakis V, Poniros N, Katsibardi K, et al. Fulminant Aeromonas hydrophila infection during acute lymphoblastic leukemia treatment. J Microbiol Immunol Infect 2012;45:154-7. [Crossref] [PubMed]
- Aldoss I, Marcucci G. More options for older patients with acute myeloid leukemia: venetoclax in combination with low dose cytarabine. Chin Clin Oncol 2019;8:S25. [Crossref] [PubMed]
- Toufexis M, Deoleo C, Elia J, et al. A link between perianal strep and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS). J Neuropsychiatry Clin Neurosci 2014;26:164-8. [Crossref] [PubMed]
- Ramesh V, Venkata NMM, Sankar J. Mixed malarial infection with pancytopenia in a child with acute lymphoblastic leukemia: an unusual presentation. Indian J Med Paediatr Oncol 2017;38:92. [Crossref] [PubMed]
- Song B, Chen Y, Fan GY. Related factors of complicated crissum infection of patients with acute leukemia. Military Medical Journal of South China 2014;28:120-2.
- Chen CY, Cheng A, Huang SY, et al. Clinical and microbiological characteristics of perianal infections in adult patients with acute leukemia. PLoS One 2013;8:e60624. [Crossref] [PubMed]
- Bal ZS, Sen S, Karapinar DY, et al. The first reported catheter-related Brevibacterium casei bloodstream infection in a child with acute leukemia and review of the literature. Braz J Infect Dis 2015;19:213-5. [Crossref] [PubMed]
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009;114:937-51. [Crossref] [PubMed]
- Ministry of Health of the People’s Republic of China. Diagnostic Criteria of Nosocomial Infection (Trial). National Medical Journal of China 2001;81:314-20.
- Tan YB, Sun DY, Song SH, et al. Risk factors and the prevention for hematogenous occupational exposure in medical staff. Chinese Journal of Nosocomiology 2017;27:221-3.
- Song SX. American Society of Colorectal Surgeons Guidelines for the Clinical Diagnosis and Treatment of Perianal Abscess, Anal fistula, and Rectovaginal Fistula. Chinese Journal of Gastrointestinal Surgery 2017;20:1437-9.
- Perazzoli C, Parra RS, Feitosa MR, et al. A Novel Severity Score Index for Febrile Neutropenic Patients with Colorectal Diseases. Gastroenterol Res Pract 2019;2019:4175960. [Crossref] [PubMed]
- Zhang X, Yu H. Matrine inhibits diethylnitrosamine-induced HCC proliferation in rats through inducing apoptosis via p53, Bax-dependent caspase-3 activation pathway and down-regulating MLCK overexpression. Iran J Pharm Res 2016;15:491-9. [PubMed]
- Li Q, Lai YM, Wang CB, et al. Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-kappa B signaling pathway. Oncol Rep 2016;35:375-81. [Crossref] [PubMed]
- Sun D, Wang J, Yang ND. Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-kappa B signaling in airway epithelial cells and asthmatic mice. Biochem Biophys Res Commun 2016;477:83-90. [Crossref] [PubMed]
- Zhu L, Pan QX, Zhang XJ. Protective effects of matrine on experimental autoimmune encephalomyelitis via regulation of ProNGF and NGF signaling. Exp Mol Pathol 2016;100:337-43. [Crossref] [PubMed]
- Wu C, Xu Z, Gai R, et al. Matrine ameliorates spontaneously developed colitis in interleukin-10-deficient mice. Int Immunopharmacol 2016;36:256-62. [Crossref] [PubMed]
- Chuang CY, Xiao JG, Chiou GC. Ocular anti-inflammatory actions of matrine. J Ocul Pharmacol 1987;3:129-34. [Crossref] [PubMed]
- Li XT. Pharmacodynamics of matrine eye drops in the treatment of bacterial keratitis and conjunctivitis. Changchun: Jilin University, 2008.
- Hu YQ, Wang YY. Research advances in anti-infective effect of matrine. Anhui Medical Journal 2016;20:1229-32.
- Vyles D, Gnagi F, Bulloch B, et al. Procalcitonin as a marker of bacteremia in patients with fever and acute lymphoblastic leukemia. Pediatr Emerg Care 2016;32:590-3. [Crossref] [PubMed]
- Ariza X, Graupera I, Coll M, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol 2016;65:57-65. [Crossref] [PubMed]
- Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol 2016;13:131-49. [Crossref] [PubMed]
- Özcan E, Saygun NI, Ilıkçı R, et al. Increased visfatin expression is associated with nuclear factor-kappa B and phosphatidylinositol 3-kinase in periodontal inflammation. Clin Oral Investig 2017;21:1113-21. [Crossref] [PubMed]
- Arai Y, Arihiro S, Matsuura T, et al. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm Bowel Dis 2014;20:1208-16. [Crossref] [PubMed]
- Abdallah DY, Jadaan MM, McCabe JP. Body mass index and risk of surgical site infection following spine surgery: a meta analysis. Eur Spine J 2013;22:2800-9. [Crossref] [PubMed]
- Willey M, Karam M. Impact of Infection on Fracture Fixation. Orthop Clin North Am 2016;47:357-64. [Crossref] [PubMed]
- Zhang D, Wei C, Yao J, et al. Interleukin-10gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17cell pathway. Exp Biol Med (Maywood) 2015;240:1622-9. [Crossref] [PubMed]