
Clinical and prognostic effects of adjuvant therapy on less advanced esophageal squamous cell carcinoma patients
Introduction
Esophageal cancer is one of the most malignant tumors in the world, with approximately 410,000 deaths every year (1), and China has the highest morbidity and mortality rates. The deaths correlated with esophageal cancer are ranked fourth among all the tumor-related deaths in China with esophageal squamous cell carcinoma (ESCC) accounting for 90% these deaths (2). Many approaches have been used for esophageal cancer treatment, including surgical resection and non-operative therapy such as chemotherapy, radiotherapy, and chemoradiotherapy. In China, the primary treatment for esophageal cancer patients is an operation, while the 5-year overall survival (OS) rate of patients with stage IIA-III ESCC treated by surgical resection alone ranges from 20.6% to 34.0% (3,4). Some studies have already confirmed the prognostic benefits the postoperative adjuvant therapy for ESCC patients with positive lymph nodes (5-7); however, there is little research on the effects of adjuvant therapy on ESCC patients with negative lymph nodes even for patients in an early stage. Some scholars proposed the prophylactic postoperative irradiation for ESCC patients (8) and found that it can reduce the metastasis rate of cervical and mediastinal lymph nodes, with patients having lymph node metastasis also receiving benefit from it. However, the prospective randomized controlled trials conducted by Zieren et al. (9) questioned this view and demonstrated that only prophylactic postoperative irradiation can reduce regional lymph node metastasis and reduce the recurrence of local tumors but does not help to prolong the survival of patients.
Meanwhile, neither chemotherapy (10,11) nor chemoradiotherapy (12,13) was reported to bring significant prognostic benefits for patients without lymph node metastasis. The nomogram has been widely applied for predicting the survival and precisely instructing medical staff by integrating a multiplicity of important factors. The nomogram has not only been demonstrated to have more accuracy in stratifying and predicting prognosis for patients with colon, breast, gastric and non-small cell lung cancers (14-17) but also has been proven to be an effective model for predicting the prognosis of ESCC patients, even after neoadjuvant therapy (18,19). However, to the best of our knowledge, no scholars have reported the effectiveness of the nomogram in less advanced ESCC patients after adjuvant therapy. Therefore, this study aimed to clarify whether it is suitable for these patients to be administrated with adjuvant therapy and if they can benefit from this treatment.
Methods
Patients
From May 2005 to May 2015, data from a total of 973 node-negative ESCC patients pathologically diagnosed as pTNM IA (pT1aN0M0G1), IB (pT1aN0M0G2-3, pT1bN0M0, and pT2N0M0G1) and IIA (pT2N0M0G2-3 cancers, pT3N0M0 cancers of the lower thoracic esophagus, and pT3N0M0G1 cancers of the upper-middle thoracic esophagus) confirmed by endoscopy and biopsy who underwent the radical esophagectomy at the Department of Thoracic Surgery, West China Hospital of Sichuan University were retrospectively reviewed. Generally, esophageal cancer is associated with the early lymphatic spread and complex distant metastasis, in which the cancer cells spread from the mucosal lymphatic ducts to drain into a rich submucosal plexus and then spread longitudinally through the dense lymphatic network (20). Although these selected patients had negative lymph node metastasis, they still had a high potential of developing lymph node metastasis, and we considered these patients to be less advanced squamous cell carcinoma patients.
The patients’ diseases were staged according to the American Joint Committee on Cancer (AJCC) Tumor, Nodes, Metastases (TNM) staging system for esophageal cancer, eighth edition (21). Patients were excluded from the study according to the following exclusion criteria: (I) patients who had undergone the palliative surgery and R1 or R2 resection; (II) patients who were lost to follow-up; (III) patients who received the neoadjuvant therapy preoperatively; (IV) patients who were diagnosed as M1 pathologically; (V) patients with fewer than 10 total dissected lymph nodes; (VI) patients pathologically defined as a higher stage than IIB-IVB; (VII) the tumor extending more than 2 cm into the gastric cardia, the longitudinal tumor length exceeding 8 cm, or the radial size exceeding 5 cm; (VIII) the tumor located less than 20 cm from the incisors; (IX) the patients were accompanied with other malignant tumors; (X) patients who were not diagnosed as ESCC. The 973 patients who presented as IA, IB, and IIA stage were enrolled in our study. The study was approved by the human participants committee of West China Hospital of Sichuan University (the ethical number: 2005-126), and all patients were informed of the risk of the operation. Permission to use their resected specimens and the written consents were obtained preoperatively. There were 776 male and 197 female patients, ranging in age from 25 to 78 years old (57.3 years old on average). The enrolled patients were divided into 4 groups according the regimen of adjuvant therapy resulting in 641, 130, 73, and 129 patients in the no-treatment, chemotherapy, radiotherapy, and chemoradiotherapy groups respectively.
Surgical procedure and pathology
In this study, the surgical approach selection depended on the patient’s images from computed tomography, magnetic resonance imaging, specific X-ray, and cervical ultrasonography; at the same time, surgeons evaluated the patient’s general condition and finally supplied them the most appropriate surgical procedure(s). Generally, the McKeown esophagogastrostomy (right thoracotomy followed by laparotomy and cervical anastomosis) with three-filed lymph node dissection could be applied for tumors in the upper, middle, and lower thoracic esophagus; in contrast, the Sweet (left thoracotomy) and Ivor-Lewis esophagogastrostomy (right thoracotomy and laparotomy) with two-filed lymph node dissection could be used for lesions in distal thoracic locations, but proximal esophageal margin was inadequate for tumors in the middle esophagus. One of the most common surgical approaches is the transhiatal esophagogastrectomy which was not considered for selection because it is hazardous and problematic in the dissection of the large, middle esophageal cancers adjacent to the trachea. As for anastomoses, ether cervical or thoracic anastomoses after esophagogastrostomy are equally safe when performed in a standardized way, which was proven by a prospective randomized trial (22). Meanwhile, the gastric conduit for reconstruction was recommended by most surgeons (23).
In regards to the surgical procedure, both minimally invasive esophagectomy (MIE) and open esophagectomy were preferred as there are no randomized trials that have assessed whether MIE improves outcomes when compared to open procedures. Meanwhile, open esophagectomy may still be favored over MIE for patients who have suffered abdominal surgery or patients with large tumors due to concerns that the gastric conduit may not be useable with difficulties performing the dissection of lymph nodes.
The dissected lymph nodes were separated from the resected esophagus and peri-esophagus tissues, which were marked to indicate the location according to the guideline of AJCC 8th TNM and Union for International Cancer Control (UICC) protocol. The mean number of dissected lymph nodes was 15 per patient [10–78]. Two experienced pathologists fixed the resected specimens, embedded them, and stained them with diaminobenzidine chromogen counterstained solution [1:50, EnVision TM Detection Kit, Gene Tech (Shanghai) Company Limited] and hematoxylin (Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China). The routine way of assessing each specimen was adopted histologically, and the pathologists documented the extent and location of metastatic lymph nodes by examining the largest cross-section of dissected lymph nodes.
Adjuvant therapy
In the present study, the patients were suggested to consult the doctors from the Oncology Department for their postoperative adjuvant therapy. The treatment options were decided according to the doctors’ experience, patients’ desire, tumor stage, tumor differentiation, and economic status of patients. Regularly, 5-fluorouracil (5-Fu) was given by continuous intravenous infusion with a dose of 800 mg/m2 over 24 hours daily on days 1 to 5 and cycled every 21 days for 4 to 6 cycles. Meanwhile, the use of cisplatin was the same as that of 5-Fu with a dose of 15 mg/m2 i.v. on days 1 to 5 and cycled every 21 days for 4 to 6 cycles. Most of the dosing schedules of the two-drug cytotoxic regimens in our study were the combinations of single drug use, and the patients who received more than 2 cycles of chemotherapy were considered as the adjuvant chemotherapy group. Furthermore, the adjuvant radiotherapy group in this study was defined as patients who were radiated by external beam radiation with a total dose of 45–50.4 Gy (1.8–2.0 Gy/d) in use of the 3D conformal radiation technique and the patients who sequentially received the radiotherapy from the first day of the first cycle of chemotherapy were regarded as the adjuvant chemoradiotherapy group. During the radiotherapy, the patients were treated in the supine position as the setup was generally more stable and reproducible; at the same time, the reproducibility was evaluated by orthogonal laser beams.
Follow-up
The patients in the present study were followed up every 3 months for the first and second year, every 6 months for the third to fifth year after the treatment, and finally, every year after the fifth year. Blood routine, gastroscopy, chest compute tomography (CT), neck and abdominal ultrasound were performed as necessary according to the patient's symptoms and physical examination, other examinations such as positron emission tomography (PET)-CT, radionuclide bone scanning, and magnetic resonance imaging (MRI) that were performed during patients’ follow-up. The tumor status (including tumor metastasis and recurrence), patients’ status (including survive and death), and the number of patients who were lost to follow-up were all recorded not only through outpatient follow-up but also through telephone and mail follow-up.
Statistical analysis
The clinicopathological characteristics among the patients in the no-treatment, chemotherapy, radiotherapy, and chemoradiotherapy groups were analyzed through Pearson’s chi-squared test or Fisher’s exact test to compare the dichotomous variables, and the Student’s t-test to compare the mean values of continuous variables. Body-mass index (BMI) was calculated by the following formula: BMI (kg/m2) = weight (kg)/height2 (m2). According to the Chinese Criteria of Weight for Adults (24), underweight, normal and overweight patients were defined as BMI <18.5, 18.5≤ BMI <24.0, and BMI ≥24.0. Meanwhile, the body surface area (BSA) [BSA (m2) =0.0061× height (cm) +0.0124× weight (kg) –0.0099], by which the appropriate dosing schedules of chemotherapy was determined (25), was categorized using the grouping criterion proposed by Vaccaro et al. (26). The total enrolled patients were divided into 2 groups according to their mean BSA value as 1.60 m2. Logistic regression analysis was performed to determine the significant independent factors related to patients with or without adjuvant therapy, and only the variables with a univariate P value <0.05 were included in the multivariate logistic regression model.
Meanwhile, the related nomogram model was adopted through the R Programming language to predict the less advanced ESCC patients for whom clinicopathological characteristics could confirm acceptance for adjuvant therapy postoperatively. The OS of each TNM stage group was revealed by Kaplan-Meier curves, and the log-rank test was used to determine the statistical significance. Multivariate survival analysis was determined through the Cox proportional hazards regression model. The nomogram model related to prognosis was also employed for predicting the 3-year and 5-year OS of less advanced esophageal cancer patients in terms of the variables with a multivariate P value <0.05 in Cox proportional hazards regression model. The discrimination and calibration of the nomogram were also assessed in our study, in which the concordance index (C-index) was applied for discrimination assessment and the calibration was evaluated by comparing the observed and predicted survival against the corresponding 3-year and 5-year cancer-specific survival probabilities calculated through the nomogram. Both bootstrap-corrected 3-year and 5-year OS were quantified by averaging the Kaplan-Meier estimates based on 200 bootstrap samples.
In addition, the group stratified analysis with regard to the total risk scores was also used in order to further demonstrate the ability of the discrimination of the nomogram. The X-tail software (http://www.tissuearray.org) was used to identify the optimal cut-off values (27).
The nomogram model was performed through the R® Version 3.4.0 (http://www.r-project.org/), and the statistical analysis was conducted by IBM® SPSS® Statistics Version 21.0. The statistical significance was regarded as a probability value <0.05 in a two-sided test.
Results
All patients
A total of 973 less advanced ESCC patients who met the inclusion criteria were finally enrolled in our study, of whom 332 received adjuvant therapy. Among them, 130 patients underwent chemotherapy, 73 patients received radiotherapy, and 129 patients received chemoradiotherapy. The median (range) age of the enrolled patients was 57 [23–89] years, while the median survival time was 22.63 (0.03–105.01) months. The clinicopathological features of all enrolled patients are presented in Table 1. Gender (P=0.029), age (P<0.001), hypertension (P=0.019), BSA (P=0.021), TNM stage (P<0.001), and recurrence (P<0.001) showed significant differences among all the enrolled patients.
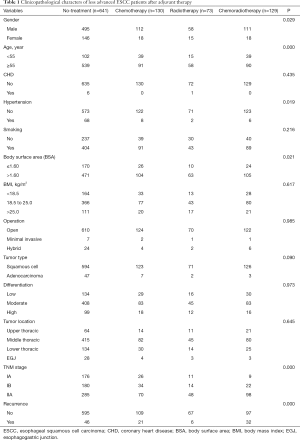
Full table
When the TNM stage was set as the dependent variable, the results of logistic regression analysis showed that the gender (P=0.032; OR =1.471, 95% CI: 1.034, 2.094), BSA (P=0.006; OR =0.625, 95% CI: 0.446, 0.875), operation (P=0.013; OR =2.227, 95% CI: 1.186, 4.183), differentiation (P=0.009; OR =0.763, 95% CI: 0.622, 0.935), tumor location (P=0.018; OR =1.255, 95% CI: 1.041, 1.513), and adjuvant therapy (P<0.001; OR =0.429, 95% CI: 0.326, 0.564) were the independent factors correlated to the less advanced ESCC patients (Table 2).
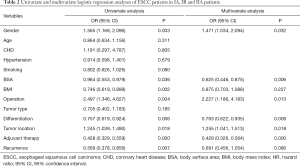
Full table
Similarly, with adjuvant therapy as the dependent variable, age (P=0.001; OR =0.551, 95% CI: 0.392, 0.773), hypertension (P=0.004; OR =0.432, 95% CI: 0.234, 0.763), BSA (P=0.004; OR =1.706, 95% CI: 1.186, 2.453), TNM stage (P<0.001; OR =1.761, 95% CI: 1.463, 2.120), and recurrence (P<0.001; OR =2.741, 95% CI: 1.777, 4.248) were demonstrated as the independent factors associated with the patients who had accepted the adjuvant therapy from the logistic regression analysis (Table 3).
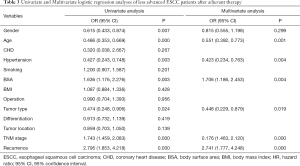
Full table
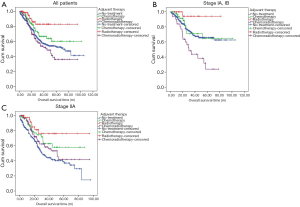
The median follow-up time (range) for all patients was 33.57 months (1.60, 105.20). The 3-year survival rates for no-treatment, chemotherapy, radiotherapy, and the chemoradiotherapy groups were 60.0%, 66.8%, 83.0%, and 54.0%, respectively, while the 5-year survival rates were well as 53.5%, 60.4%, 83.0%, and 36.3%, respectively. The Kaplan-Meier curves showed that patients in the radiotherapy group had the most significantly better prognosis when compared with that of patients in the no-treatment (P=0.001) and chemoradiotherapy groups (P<0.001), though the difference between the radiotherapy and chemotherapy groups was not significant (P=0.055). At the same time, the patients in the chemotherapy group received a significantly better prognosis than the patients in the chemoradiotherapy group (P=0.019). The OS rates in the chemotherapy group and chemoradiotherapy group tended to be higher and lower than that of patients who did not receive the adjuvant therapy respectively, while the differences were not significant (P=0.063 and P=0.194, respectively) (Figure 1A). From the Cox proportional hazards regression analysis, BSA (P=0.027; HR =0.749, 95% CI: 0.579, 0.968), BMI (P=0.014; HR =0.793, 95% CI: 0.659, 0.955), recurrence (P<0.001; HR =2.890, 95% CI: 2.228, 3.749), and TNM stage (P<0.001; HR =1.374, 95% CI: 1.175, 1.606) were demonstrated as the independent prognostic factors for less advanced esophageal cancer patients.
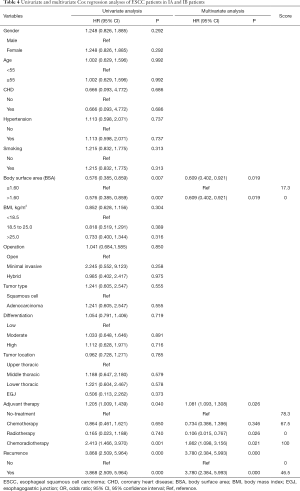
IA and IB patients
The median follow-up time (range) and the median survival time for IA and IB patients were 33.50 months (1.60, 105.20) and 24.33 months. The 3-year survival rates for no-treatment, chemotherapy, radiotherapy, and chemoradiotherapy group were 71.2%, 71.1%, 94.1%, and 43.8%, respectively, and the 5-year survival rates for these 4 groups were 65.7%, 64.6%, 94.1%, and 24.0%, respectively. From the Kaplan-Meier curves, we concluded that no significant survival difference was found between the patients in the no-treatment and chemotherapy group (P=0.656); however, patients who accepted the radiotherapy still obtained the best prognosis when compared with the other 3 groups, while the OS rate in chemoradiotherapy group was the lowest (Figure 1B). Meanwhile, BSA (P=0.019; HR =0.609, 95% CI: 0.402, 0.921), recurrence (P<0.001; HR =3.780, 95% CI: 2.384, 5.993) and adjuvant therapy (P=0.026; HR =1.081, 95% CI: 1.093, 1.308) were drawn as the independent prognostic factors for IA and IB patients from the Cox proportional hazards regression analysis (Table 4).
Full table
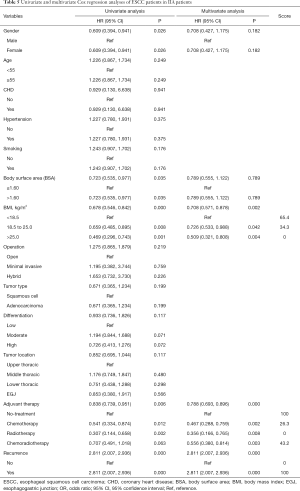
IIA patients
A total of 502 patients were diagnosed as stage IIA according to the TNM stage system. The median follow-up time (range) for these patients was 33.57 months (1.8, 96.23) months, and the median survival time was 20.63 months. Among them, 285 patients did not accept the adjuvant therapy postoperatively, and there were 70, 48, and 98 patients who received chemotherapy, radiotherapy, and chemoradiotherapy, respectively. The 3-year survival rates for these 4 groups were 45.6%, 62.8%, 76.1%, and 58.3%, respectively, while their 5-year survival rates were 38.8%, 58.0%, 76.1%, and 41.8%, separately. The Kaplan-Meier curves showed that the prognosis of patients in the chemotherapy, radiotherapy and chemoradiotherapy groups was better than that of patients who did not receive the adjuvant therapy. Among them, the OS rates of both the chemotherapy and radiotherapy groups were significantly higher than that of the patients in the no-treatment group (P=0.001 and P=0.010, respectively), and the prognosis of radiotherapy group tended to be better than that of the chemotherapy group, while the difference was not significant (P=0.210). Similarly, the OS rate of the chemoradiotherapy group tended to be lower than that of the chemotherapy group but higher than that of no-treatment group; however, neither of these differences was significant (P=0.405 and P=0.064, respectively) (Figure 1C). Furthermore, BMI (P=0.002; HR =0.708, 95% CI: 0.571, 0.878), recurrence (P<0.001; HR =2.811, 95% CI: 2.007, 2.936), and adjuvant therapy (P<0.001; HR =0.788, 95% CI: 0.693, 0.896) were demonstrated to be independent prognostic factors of the IIA patients (Table 5).
Full table
Nomogram for IA and IB patients
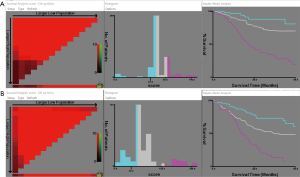
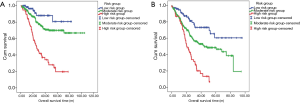
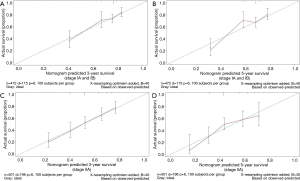
Based on these significant independent factors and selected variables with hazard ratios, a nomogram for predicting 3-year and 5-year survival in IA and IB patients was established. The nomogram showed that adjuvant therapy contributed the most to prognosis, followed by recurrence rate, while BSA showed the smallest effect on survival rate (Figure 2). Each variable was given a score on the point scale, and we were able to predict the probability of the 3-year and 5-year survival by adding up the total score corresponding to the bottom scale. The assessment of the nomogram was performed subsequently with regards to discrimination and calibration. The C-index value, which was used to evaluate the discrimination of the nomogram, was 0.665, with 95% CI ranging from 0.569 to 0.761. In addition, the IA and IB ESCC patients were stratified into 3 risk groups according to the total risk scores (total risk scores: low-risk group: ≤67.5, moderate-risk group: 67.5–95.6, high-risk group >95.6) (Figure 3). The 5-year OS of each group was 80.7%, 70.0%, and 19.6%, respectively (low-risk group versus moderate-risk group: P=0.044; moderate-risk group versus high-risk group: P<0.001) (Figure 4). The calibration plot of the nomogram is shown in Figure 5, which illustrates that the predicted 3-year and 5-year survival probabilities for IA and IB patients agreed well with the actual observations.

Nomogram for IIA patients
A nomogram for predicting 3-year and 5-year survival in IIA patients was constructed based on the results of the Cox proportional hazards regression analysis, which showed that the adjuvant therapy had the largest contribution to prognosis, with recurrence ranking second. The BMI had the smallest effect on the prognosis of IIA patients (Figure 2). Points for these independent factors were assigned according to their coefficients, and the probability of the 3-year and 5-year OS were determined by the total number of points, which was the sum of all points. With regards to the discrimination of the nomogram, the C-index was 0.645, with a 95% CI ranging from 0.567 to 0.723.
The patients were also divided into 3 risk groups with respect to the total risk scores (total risk scores: low-risk group: ≤43.2; moderate-risk group: 43.2–126.3; high-risk group >126.3) (Figure 3). The 5-year OS of each group was 60.4%, 48.5%, and 6.7%, respectively (low-risk group versus moderate-risk group: P=0.001; moderate-risk group versus high-risk group: P<0.001) (Figure 4). Calibration curves were built in order to compare the nomogram that predicted the probabilities of OS with the actual survival at years 3 and 5, from which we observed a high degree of similarity between the observed and the estimated rate (Figure 5).
Discussion
It is reported that the surgical approaches alone may not improve the prognosis of esophageal cancer patients, with the postoperative OS rate between 10–40% (28,29). Furthermore, recent research on other modalities for esophageal cancer have mainly focused on neoadjuvant therapy, especially neoadjuvant chemoradiotherapy (NCRT) (30), and have demonstrated that patients might benefit from the administration of NCRT when compared with surgical approaches alone. Meanwhile, the results were corroborated by the recent randomized controlled trial of CROSS (Chemoradiotherapy for Esophageal Cancer, Followed by Surgery Study) conducted by Shapiro et al. (31). However, few studies have reported the effects of the adjuvant therapy after surgery for esophageal cancer patients, including for less advanced esophageal cancer patients. Therefore, this study aimed to evaluate the prognostic effect of adjuvant therapy for patients with esophageal cancer in IA, IB, and IIA stage.
It is recommended in the National Cancer Care Alliance (NCCA) guideline (32) that the patients with ESCC do not need adjuvant therapy after a complete resection of the tumor, regardless of the depth of invasion or lymph node metastasis. However, there are still many studies that have proven the prognostic benefits of adjuvant therapy brought to esophageal cancer patients with lymph node metastasis. Li et al. (33) retrospectively reviewed 408 ESCC patients, and they concluded that the postoperative adjuvant therapy was associated with a better prognosis for middle mediastinal ESCC patients with metastatic lymph nodes. Meanwhile, the multicenter randomized controlled trial conducted by Ando et al. (11) (JCOG9204) examined the effect of the postoperative adjuvant therapy in ESCC patients, and found that the 5-year disease-free survival (DFS) rate of ESCC patients who had surgery alone or surgery with postoperative adjuvant therapy was 45% and 55%, respectively (P=0.037); a similar result was also seen in the 5-year OS rate between the 2 groups (52% and 61% respectively).
From the present study, we found that adjuvant therapy was an independent factor correlated to less advanced ESCC patients, and that radiotherapy provided the best survival benefits to the patients. Meanwhile the worst prognosis was seen in patients in IA and IB stage who were given chemoradiotherapy, and the patients in IIA also had fewer prognostic benefits from it. In 1991, the Ténière et al. study (34) was first to report that postoperative radiotherapy did not improve the prognosis of ESCC patients through a prospective multicenter controlled trial. Meanwhile, the RCT trials performed by Fok et al. (13) also demonstrated that the overall median survival of patients without postoperative radiotherapy was significantly longer than those who accepted adjuvant therapy (15.2 vs. 8.7 months P=0.02). However, the shorter survival of the patients who received postoperative radiotherapy might not be attributed to radiotherapy itself; on the contrary, irradiation-related death and the early appearance of metastatic diseases were correlated to it (13). Yang et al. (35) was first to research the survival benefits of postoperative radiotherapy for pT3N0M0 ESCC patients and found that the 5-year survival rate and the 5-year DFS rate in postoperative radiotherapy group were significantly higher than those in the surgery alone group (75.2% vs. 58.5%, P=0.004 and 73.3% vs. 49.2%, P=0.001), and the results were confirmed in the matched samples through propensity score-matching analysis. At the same time, Zhu et al. (2) had comparable results as Yang et al. (35).
Although there is always controversy between different studies, there are several reasons why our research can be considered accurate. First, a lower recurrence rate was a benefit brought to less advanced ESCC patients by radiotherapy. Earlier studies reported that the overall recurrence of ESCC patients in stage pT2-3M0N0 ranged from 33.3% to 41.6% (36,37), and significantly higher lymph node recurrence rates were seen in ESCC patients with metastatic lymph nodes (28,37,38). Kasai et al. (8) once reported that lymph node recurrence mostly occurs in the neck and upper mediastinum, which can be attributed to the non-resectable small metastasis on the lymph nodes in these regions. Prophylactic postoperative irradiation was proposed in 1970, and it was demonstrated to be especially effective in patients without lymph node metastasis with a 5-year survival rate of 87.5%. Meanwhile, a prospective randomized study which focused on the postoperative radiation therapy conducted by Zieren et al. (9), also proposed that postoperative radiotherapy might reduce the rate of tumor recurrence and regional lymph node metastasis but with no benefit to the OS rate. In our study, the recurrence rates for the chemotherapy, radiotherapy, and chemoradiotherapy groups were 16.16%, 8.22%, and 24.8% (P<0.001), respectively, indicating that the ESCC patients without lymph node metastasis in radiotherapy group had the lower recurrence rate with recurrence being the independent prognostic factor in our study. Therefore, whether it is suitable to administer prophylactic postoperative irradiation to the less advanced ESCC patients is still unclear, and a large-scale prospective cohort study is required to confirm this.
Second, chemotherapy or chemoradiotherapy may not kill the micro-metastasis of tumors directly when compared with radiotherapy. In our study of less advanced ESCC patients, the 3-year survival rates for chemotherapy, radiotherapy, and chemoradiotherapy group were 66.8%, 83.0%, and 54.0%, respectively, compared with that of 5-year survival rates which were 60.4%, 83.0%, and 36.3%, respectively. The results are similar to that of Liu et al.’s study (39), in which the 3-year and 5-year survival rates for ESCC patients in the IIA stage were 73.33% and 53.33% in radiotherapy group respectively, in contrast to 64.28% and 35.71% (X2=6.74, P<0.01) in chemotherapy group. Meanwhile, the rate of lymph node metastasis to the supraclavicular region, mediastinal region, and thoracic vertebra after radiotherapy was 17.57%, 14.86%, and 2.70%, respectively, compared to 23.29%, 32.88%, and 5.48% in chemotherapy group, respectively (P<0.01). It is generally accepted that operation is the priority for ESCC patient treatment. However, the operation itself also has certain destructive effects. In particular, the operation causes certain damage to local blood vessels and lymphatic vessels of the mediastinum and esophageal bed; meanwhile, the local blood and lymph circulation system is destroyed and blocked. Under normal circumstances, this surgical injury will not have a significant impact on the postoperative physiology of the patient, but it will have different effects on the efficacy of postoperative radiotherapy or chemotherapy. Micro-metastases remaining after esophagectomy is also a source of recurrence (8). Owing to the surgical injury, the blood vessels or the lymphatic vessels of residual tumors or the micro-metastasis are destroyed or blocked, and there is a higher possibility of metastasis existing in tumor invasive growth rather than vascular invasion. That is, insufficient blood or lymphatic supply to the tumor stimulates the invasive growth of the tumor. Nevertheless, radiotherapy may irradiate the residual lesions or the remaining micro-metastasis around the primary tumor in the irradiation area directly, which is not affected by the surgical approaches; therefore, it has a definite curative effect in killing the residual tumor, and reducing the local, mediastinal, and supraclavicular recurrence rate (40).
The nomogram model applied in our study was confirmed to be a good evaluation model for estimating the prognosis of patients with tumors and has been used to evaluate many malignancies. Furthermore, some studies have reported the nomogram model was more reliable than the traditional staging system (18,19). Owing to the different prognostic effects of postoperative chemoradiotherapy in stage I and IIA ESCC patients, we constructed 2 nomogram models for both stages I and IIA ESCC patient groups. The clinicopathological factors, which were confirmed as the independent prognostic factors through multivariate Cox regression analysis, were taken into consideration. The prognostic nomograms we constructed for both stages I and IIA ESCC patients showed there to be an acceptable agreement between the prediction probabilities and actual observations in terms of the 3-year and 5-year OS rates in the 2 groups, which were stratified according to the total risk scores. Additionally, the specific survival rates for specific ESCC patients can be drawn through the nomogram with regards to the included clinicopathological factors, which, to a degree, make it easier for surgeons to tailor personalized treatment schemes for patients (17).
There are some limitations to our study that should be addressed. First, few studies have reported the effects of postoperative adjuvant on less advanced ESCC patients; on the contrary, some researchers, even the prospective trials conducted in China, had similar results as we did. Therefore, an international randomized controlled trial is required to confirm our results. Second, in our hospital, the patients who received the surgery and the adjuvant therapy were from different departments; therefore, it was hard for us to postoperatively monitor each of the patients adjuvant-customized by the doctor of the Oncology Department, and some of the patients could not be traced afterward as they did not follow the postoperative adjuvant scheme consecutively or were lost in follow-up. Although not many patients received the adjuvant therapy in our study, the included patients were followed up strictly with respect to their adjuvant therapy scheme, duration of adjuvant therapy, and compliance with adjuvant therapy. The prognostic effects of adjuvant therapy to the less advanced ESCC patients were authentically demonstrated, and relatively strong conclusions could be drawn from the observation of a sufficient number of events.
In conclusion, among less advanced ESCC patients, adjuvant therapy was not only identified as the independent factor but also proved to be of importance in the prognosis of these patients. Additionally, radiotherapy consistently provided the best prognosis to patients when compared to chemotherapy and chemoradiotherapy. However, ESCC patients in IA and IB stage had the worst prognosis after receiving chemoradiotherapy, and still, small survival benefits of chemoradiotherapy were seen in IIA stage ESCC patients. Nomogram models for I and IIA stage ESCC patients were also constructed, and each of them was evaluated to stratify and predict the specific survival rate. Our results still need to be confirmed through an international randomized controlled trial.
Acknowledgments
The authors thank the Department of Pathology of West China Hospital, Sichuan University, China, for the substantial work in diagnosing and pathologically examining the dissected tissues and lymph nodes pathologically.
Funding: This study was supported by the Key Innovation Team of Shanxi 1331 Project approved by Shanxi Education Department and Shanxi Finance Department (2017 #12) and the Service Industry Innovation Discipline Group “Integrated Prevention and Treatment of Gastrointestinal Tumors” Project approved by Shanxi Education Department (2018 #4).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the human participants committee of West China Hospital of Sichuan University (the ethical number: 2005-126). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel R, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zhu D, Bin Li B, Wang C, et al. A retrospective study of the effect of postoperative adjuvant therapy on patients with locally advanced pT3N0M0 esophageal squamous cell carcinoma. Chin J Clin Oncol 2018;45:228-31.
- Ping Y, Zhang Y, Du X, et al. Surgical treatment experiences of 20 000 cases of esophageal and cardiac cancer. The First International Symposium on Esophageal Cancer and the Seventh National Symposium on Esophageal Cancer in China, 2005:167-71.
- Shao L, Gao Z, Xu J, et al. Surgical treatment of 15707 cases of esophageal cancer and cardiac cancer: a summary of the prevention and treatment of esophageal cancer in Henan Province. The First International Symposium on Esophageal Cancer and the Seventh National Symposium on Esophageal Cancer in China, 2005:40-5.
- Wong AT, Shao M, Rineer J, et al. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann Surg 2017;265:1146-51. [Crossref] [PubMed]
- Xu Y, Liu J, Du X, et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol 2013;8:116. [Crossref] [PubMed]
- Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol 2010;5:244-50. [Crossref] [PubMed]
- Kasai M, Mori S, Watanabe T. Follow-up results after resection of thoracic esophageal carcinoma. World J Surg 1978;2:543-51. [Crossref] [PubMed]
- Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444-9. [Crossref] [PubMed]
- Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg 1997;114:205-9. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]
- Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 2008;26:380-5. [Crossref] [PubMed]
- Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol 2012;30:2837-43. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Deng W, Wang Q, Xiao Z, et al. A prognostic nomogram for overall survival after neoadjuvant radiotherapy or chemoradiotherapy in thoracic esophageal squamous cell carcinoma: a retrospective analysis. Oncotarget 2017;8:41102-12. [Crossref] [PubMed]
- Cao J, Yuan P, Wang L, et al. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy. Sci Rep 2016;6:26684. [Crossref] [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Lymph node metastasis and recurrence in patients with a carcinoma of the thoracic esophagus who underwent three-field dissection. World J Surg 1994;18:266-72. [Crossref] [PubMed]
- Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springrt, 2017:185-202.
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Urschel JD, Blewett CJ, Bennett WF, et al. Handsewn or stapled esophagogastric anastomoses after esophagectomy for cancer: meta-analysis of randomized controlled trials. Dis Esophagus 2001;14:212-7. [Crossref] [PubMed]
- The Ministry of Health released the criteria for judging the fat and thinness of Chinese people. Quality Exploration 2010:21.
- Lemoine L, Thijssen E, Carleer R, et al. Body surface area-based vs concentration-based perioperative intraperitoneal chemotherapy after optimal cytoreductive surgery in colorectal peritoneal surface malignancy treatment: COBOX trial. J Surg Oncol 2019;119:999-1010. [Crossref] [PubMed]
- Vaccaro CA, Vaccarezza H, Rossi GL, et al. Body surface area: a new predictor factor for conversion and prolonged operative time in laparoscopic colorectal surgery. Dis Colon Rectum 2012;55:1153-9. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11. [Crossref] [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72; discussion 572-3. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Li L, Zhao L, Lin B, et al. Adjuvant Therapeutic Modalities Following Three-field Lymph Node Dissection for Stage II/III Esophageal Squamous Cell Carcinoma. J Cancer 2017;8:2051-9. [Crossref] [PubMed]
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Yang J, Zhang W, Xiao Z, et al. The Impact of Postoperative Conformal Radiotherapy after Radical Surgery on Survival and Recurrence in Pathologic T3N0M0 Esophageal Carcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2017;12:1143-51. [Crossref] [PubMed]
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [Crossref] [PubMed]
- Visbal AL, Allen MS, Miller DL, et al. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg 2001;71:1803-8. [Crossref] [PubMed]
- Liu Q, Cai XW, Wu B, et al. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapy. PLoS One 2014;9:e97225. [Crossref] [PubMed]
- Liu J. Clinical observation of postoperative radiotherapy and chemotherapy for 147 patients with esophageal carcinoma. Chin J Clin Oncol 2009;36:859-61.
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82-90. [Crossref] [PubMed]


