Might definitive local therapy of the primary tumor improve the survival benefits of metastatic prostate cancer?—evidence from a meta-analysis
Introduction
Prostate cancer (PCa) is the second most frequent type of malignancy among the male population worldwide, with 164,690 newly estimated cases and 29,430 newly estimated deaths in the United States, 2018 (1). Due to the aging population, it was foreseeable that the incidence of PCa would substantially increase in the following years, which could make it a huge health care problem in China (2). Generally, the major interventions for men with clinically localized PCa are radical prostatectomy (RP) and radiation therapy (RT), and good outcomes have been verified (3,4). However, in metastatic cases, the recommended therapy by European Association of Urology (EAU) guidelines was androgen deprivation therapy (ADT) with or without chemotherapy (5). Recently, accumulating studies had successfully confirmed the significant improvement of survival benefit of treatment of the primary tumor in metastatic cancers such as ovarian and renal cell carcinoma (6,7), and in which two aspects of the role highlighted, reducing the overall tumor burden and interrupting the re-seeding of the primary tumor (8,9). Nowadays, the traditionally seldom involved role of LT in the treatment of metastatic prostate cancer (mPCa) had gained a lot of interest.
Metastases were responsible for most of the deaths among cancer patients, whereas few effective treatments could be available (10). Furthermore, the factors regulating the development of metastases had not been fully elucidated. The accumulating data had suggested that the definitive treatment of the primary tumor could suppress systemic disease progression and improve survival (11,12). Currently, the treatment regimens for mPCa had advanced greatly and patients could receive LT (RP or RT) or no local therapy (NLT) such as systemic therapies (ADT with or without chemotherapy), based on a more comprehensive evaluation of patient’s general condition and wishes, the extent of the metastases, the treatment technique, the treatment response and so on (13). However, optimal treatment for mPCa remained a clinical dilemma.
Despite enthusiasm of LT for mPCa, previous studies had not reached a clinical consensus. Hence, this meta-analysis was conducted to shed light on the merits of such an approach based on available data and meanwhile three clinical outcomes such as OS, cancer-specific mortality (CSM) and all-cause mortality (ACM) were calculated. The results of ours were anticipated to provide some references for clinical work.
Methods
Search strategy
A comprehensive and systematic literature review was performed by using multiple search engines (PubMed, EMBASE, Web of Science) to identify eligible studies, up to May 2019. The search strategy mainly consisted of two parts (different treatments and mPCa), using the following keywords in combination with Medical Subject Headings (MeSH) terms and text words: “local therapy” or “LT” or “radical prostatectomy” or “cytoreductive prostatectomy” or “RP” or “radiation therapy” or “radiotherapy” or “RT” or “androgen-deprivation therapy” or “hormonal therapy” or “chemohormonal therapy” or “ADT” or “metastatic prostate neoplasms” or “metastatic prostate cancer” or “metastatic neoplasms of the Prostate” or “metastatic cancer of the Prostate” or “mPCa”. Additional studies were identified manually by searching relevant reviews and the reference list of original articles.
Inclusion and exclusion criteria
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria were used for article selection, which was performed by two investigators (14). Articles enrolled in this study should meet the following criteria: (I) titles were screened for manuscripts written in the English language; (II) original studies comparing LT or NLT for mPCa; (III) sufficient data should be available; (IV) the clinical outcomes such as OS, ACM or CSM should be involved at least one. Studies would be excluded if they met the following criteria: (I) duplicates or reviews or letters or case reports or comments or editorials; (II) unrelated to the topic of this study; (III) lack of sufficient data.
Data extraction
Two independent reviewers (M Xiao and R Cong) participated in the selection procedure of eligible studies, according to the inclusion and exclusion criteria. The titles of the articles were first reviewed to ascertain whether they might potentially fit the inclusion criteria. After assessing the abstracts, a more thorough subsequent assessment was performed by looking at the full-text. Studies without primary data (such as reviews, letters or commentaries) were excluded but were examined to ensure that relevant citations had been included. Disagreements between reviewers were resolved by discussion and consensus with a third reviewer (Q Zhang).
The extracted data elements were included as follows: (I) the first author’s name and year of publication; (II) the treatment and control arm; (III) study design and number of patients; (IV) the clinical outcome (OS, CSM, ACM) and corresponding hazard ratio (HR) with 95% confidence intervals (CIs). If HRs and 95% CIs were not directly given, they were calculated based on the reported Kaplan-Meier curve and the results were entered into a data extraction sheet by previously described method and it had been approved by all reviewers (15,16).
Quality assessment
This meta-analysis was strictly performed according to the PRISMA statement and the level of evidence was rated for each study included. The quality of each study was determined using the Newcastle-Ottawa Scale (NOS) for non-randomized controlled trials (RCTs) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm) (17). Its detailed information were as follows: (I) representativeness of the exposed cohort; (II) selection of the non-exposed cohort; (III) ascertainment of exposure; (IV) outcome of interest not present at start of study; (V) control for important factor or additional factor; (VI) assessment of outcome; (VII) follow-up long enough for outcomes to occur; (VIII) adequacy of follow up of cohorts. In addition, the whole quality score was ranged between 0 and 9. A total score of 5 or fewer stars was considered as low, 6–7 was considered as intermediate, and 8–9 was regarded as high quality. The detailed ranking of eligible studies enrolled in this article was displayed in Table 1.

Full table
Statistical analysis
Data were extracted from eligible studies to shed light on the effectiveness of LT for mPCa and it was presented in the form of the HR with 95% CI. The random-effects model (DerSimonian-Laird method) or the fixed-effects model (Mantel-Haenszel method) was used for meta-analysis according to the heterogeneity among the involved studies (18). Moreover, the heterogeneity test for pooled HRs was defined and quantified by Cochran Q test or Higgins I2 statistic. If significant heterogeneity was observed (P<0.10 or I2>50%), a random-effects model was utilized; otherwise, the fixed-effects model was applied. Besides, if significant heterogeneity existed, we would minimize the influence of by classifying the enrolled studies into subgroups. Meanwhile, sensitivity analysis was conducted to access the stability of results by deleting one single study each time to reflect the impact of the individual to overall. Furthermore, publication bias was estimated by using Egger’s linear regression test with a funnel plot (19). All P values were calculated by a two-sided test, and a P value of less than 0.05 was considered statistically significant. All statistical analyses were conducted with Stata12 (StataCorp LP, College Station, Texas, USA), and Microsoft Excel (V.2007, Microsoft Corporation, Redmond, Washington, USA).
Results
Characteristics of enrolled studies
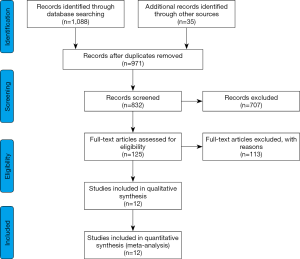
A total of 1,123 eligible studies were identified from a primary literature survey by searching online databases PubMed, EMBASE, Web of Science and 971 remained after removal of duplications. After assessment of the titles and abstracts, 846 records were excluded because they were reviews, letters, commentaries, non-English articles, did not use human subjects, or were not relevant to the current analysis. Of the remaining 125 studies under full-text articles evaluation, 113 did not contain sufficient survival data (HRs or survival curves), nor even one of the three clinical outcomes (OS, CSM or ACM). Finally, 12 studies were considered to be eligible and enrolled in this meta-analysis (12,20-30) (Figure 1).
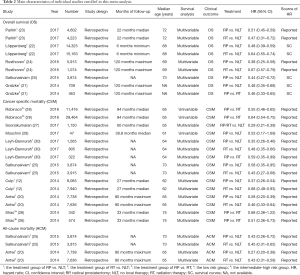
Detailed information about all these 12 involved studies with 78,864 participants was summarized in Table 2 and they were all retrospective cohort study. Each ranking of all these enrolled studies were presented in Table 1, from which we could easily find that the whole quality scores were ranged between 6 and 8. In other words, it could be regarded as intermediate-high quality. Furthermore, three clinical outcomes (OS, CSM or ACM) were calculated simultaneously.
Full table
OS associated with LT for mPCa
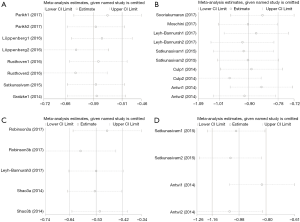
A total of five studies containing eight comparisons contributed to the analysis of OS. The results revealed a prognostic role of LT for mPCa on OS by random-effects model based on moderate heterogeneity (P=0.015, I2=59.7%). LT for mPCa was related to enhanced OS (pooled HR =0.53, 95% CI: 0.47 to 0.61) (Figure 2A). When classifying these enrolled studies into subgroups based on treatment, the heterogeneity was further reduced. Meanwhile, RP or RT vs. NLT was respectively associated with longer OS (pooled HR =0.49, 95% CI: 0.44 to 0.54, I2=0.0%, P=0.741; pooled HR =0.64, 95% CI: 0.56 to 0.72, I2=15.4%, P=0.306) (Figure 2B).

CSM associated with LT for mPCa
In the analysis of CSM, a total of six studies containing 10 comparisons contributed to it. As similar results as OS, it indicated the positive role of LT for mPCa by random-effects model depending on moderate heterogeneity (P=0.004, I2=63.1%). Our results successfully demonstrated that decreased CSM was associated with LT for mPCa (pooled HR =0.42, 95% CI: 0.34 to 0.51) (Figure 2C). It seemed to display a significant heterogeneity. Hence, subsequently stratified analysis was conducted to further minimize the heterogeneity. We could find in Figure 2D that the heterogeneity had been reduced significantly. No matter how RP or RT compared with NLT, it was correlated with a lower CSM (pooled HR =0.37, 95% CI: 0.29 to 0.46, I2=35.2%, P=0.187; pooled HR =0.51, 95% CI: 0.42 to 0.63, I2=27.0%, P=0.250).
CSM associated with RP vs. RT
A total of five different comparisons shed light on the efficacy of RP vs. RT in terms of CSM in the fixed-effects model with no heterogeneity (P=0.653, I2=0.0%). Our results showed that RP presented its definite superiority in comparison with RT (pooled HR =0.59, 95% CI: 0.53 to 0.66) (Figure 2E). In other words, patients with mPCa could gain more survival benefits from RP than RT in the case of CSM.
ACM associated with mPCa
All these enrolled four comparisons demonstrated the prognostic role of LT for mPCa. LT for mPCa was correlated with decreased ACM (pooled HR =0.37, 95% CI: 0.31 to 0.45, I2=49.4%, P=0.115) in the fixed-effects model (Figure 2F). Subsequently stratified analysis shed light on that no matter how RP or RT compared with NLT, it was linked to a lower ACM (pooled HR =0.31, 95% CI: 0.23 to 0.40, I2=56.4%, P=0.130; pooled HR =0.44, 95% CI: 0.34 to 0.56, I2=0.0%, P=0.856) (Figure 2G).
Sensitivity analysis
Sensitivity analysis was performed to evaluate the stability of our results by means of deleting one single study each time to reflect the impact of the individual to overall. As indicated in Figure 3, no single study significantly influenced the pooled HR or the 95% CI in the assessment of sensitivity analysis of all three clinical endpoints (OS, CSM or ACM). In other words, our results might be robust.
Publication bias
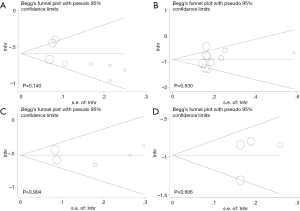
Publication bias was examined by Begg’s and Egger’s test with a funnel plot. In the pooled analysis of OS, the P value of Begg’s or Egger’s test was 0.711, 0.140 respectively (Figure 4A). In the same analysis of CSM associated with LT for mPCa, the P value of Begg’s test was 0.592 and the P value of Egger’s test was 0.530 (Figure 4B). In terms of CSM associated with RP vs. RT, the P value of Begg’s test was 0.806 and the P value of Egger’s test was 0.904 (Figure 4C). In the similar case of ACM, the P value of Begg’s or Egger’s test was 0.734, 0.606 separately (Figure 4D). We could easily find that all the P values were above 0.05, which indicated that there was no significant bias in this meta-analysis.
Discussion
PCa is the second most commonly diagnosed malignancy and the second leading cause of cancer-related death in the United States of the male population. Since the application of prostate-specific antigen (PSA) plus digital examination of the rectum (DRE) in screening, more and more men were found metastasis at initial diagnosis (31). Usually, in the case of mPCa, ADT with or without chemotherapy was currently the gold standard treatment, based on the guidelines of the EAU and the National Comprehensive Cancer Network (NCCN) (32). As for LT, most urologists reached a consensus that RP or RT were two effective interventions for localized PCa (13). However, these therapies were seldomly involved in the treatment of mPCa. With the development of surgical techniques and the survival benefit of LT in other metastatic disease such as ovarian and renal cell carcinoma, whether it was equally effective in the treatment of mPCa, had gained more and more interest of urologists. This meta-analysis was performed to shed light on its effectiveness in a broader range.
As far as we were concerned, this was the largest meta-analysis to demonstrate its prognostic role of LT in the treatment of mPCa. Twelve eligible studies with 78,864 participants, containing 28 different comparisons were ultimately enrolled in this article and three clinical outcomes (OS, CSM and ACM) were calculated simultaneously. Compared with NLT, LT (RP or RT) showed its definite superiority in improving OS and cutting down CSM or ACM. In addition, RP was related to a decreased CSM when compared with RT.
Consistent with our results, Antwi et al. demonstrated that definitive LT (either RP or brachytherapy) of the primary tumor could significantly improve survival in men with mPCa (20). Culp et al. drew the conclusion that LT appeared to confer a survival benefit (12). Therein, three aspects of the role highlighted, interrupting the re-seeding of the primary tumor, increasing tumor response to systemic chemotherapy or reducing the overall tumor burden (33,34). Although, we had successfully demonstrated the feasibility and survival benefit of LT for mPCa in improving OS or decreasing CSM, ACM, not all patients were suitable for it. Previous studies by Fossati et al. and Wang et al. revealed that patients with a relatively lower level of tumors and better general health seemed to benefit the most (35,36). Moreover, in a short period of time, no survival benefits have been observed for patients treated with RP compared with patients treated with androgen deprivation treatment (29).
There were also several advantages in our study. As a powerful tool, meta-analysis could provide more reliable results than a single study, especially in explaining controversial conclusions (37). Hence, this article was conducted to clarify the relationship between different therapeutic regimens of mPCa in a larger range of the population and it was strictly performed according to the PRISMA statement. Meanwhile, the heterogeneity of throughout this article was moderate to low and it could be further minimized by stratified analysis. Furthermore, the results of sensitivity analysis and publication bias indicated the stability of our conclusions. Although several similar meta-analyses already published (36,38,39), this study was the largest meta-analysis to demonstrate its prognostic role of LT in the treatment of mPCa and the first time to analyze ACM associated with mPCa between RP, RT and NLT.
As emphasized by Leyh-Bannurah et al., risk stratification is an important factor in the consideration of the therapeutic effect of LT. Clinical variables consisting of age, race, marital status, biopsy Gleason score, clinical tumour, nodes, and metastatic substages, were subsequently utilized in a risk stratification scheme of ≤1 vs. ≥2 risk factors. Leyh-Bannurah et al. revealed that LT was less effective in patients with ≥2 risk criteria compared with those with ≤1 and meanwhile the CSM benefit was not observed in patients with ≥2 risk criteria (30). Coupled with previous research findings, the general conditions of patients and the risk stratification before treatment were two major factors affecting the therapeutic effects.
The mechanism by which LT plays in the treatment of mPCa remained unknown, however, there are several hypotheses. On the one hand, eradication of the primary tumour eliminate the source of cytokine signalling which is the predominant source of metastasis (40). On the other hand, the primary tumour can act as the source of circulating tumour cells which have the potential of “self-seeding” of the primary tumour (8). Last but not least, eradication of self-renewing progenitor cells persisting after ADT, which leads to an immature luminal and androgen receptor low phenotype, can propagate adenocarcinoma (41).
To some extent, several limitations should be taken into account before comprehensively understanding this article. Firstly, although meta-analyses could be utilized as a robust statistical tool, controversies related to its inherent nature had been widely recognized. Secondly, all of the involved studies were retrospective, which could not have the same statistical power as RCTs. Thirdly, we did not take the “surgical technique and operator” factor into account, and we were unable to perform a subgroup analysis based on surgical technique (such as laparoscopic vs. robotic vs. open) and its relevant complications.
To sum up, the aim of treatment was to extend life and to relieve symptoms while ensuring the best possible quality of life. Our work had shed light on the feasibility and the survival benefit of LT for mPCa and meanwhile RP presented its superiority in comparison of RT. Meanwhile, several limitations should be taken into consideration simultaneously, when fully understanding our results.
Conclusions
Taken together, our results suggested the positive role of LT (RP or RT) for mPCa and we also shed light on the survival benefits in terms of OS, CSM or ACM. Besides, when comparing different treatments of LT, RP was linked to a decrease CSM, in the comparison of RT. All these aforementioned data were statistically different. Meanwhile, the general conditions of patients and the risk stratification before treatment were two major factors affecting the therapeutic effects. Hopefully, our results could provide some references for clinical work. Larger sample sizes with more strictly RCTs were required to provide more high-quality data.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.21). The authors have no conflicts of interest to declare.
Ethical Statements: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Yang Y, Ma Y, Sheng J, et al. A multicenter, retrospective epidemiologic survey of the clinical features and management of bone metastatic disease in China. Chin J Cancer 2016;35:40. [Crossref] [PubMed]
- Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Arch Intern Med 2010;170:1256-61. [Crossref] [PubMed]
- Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw 2010;8:240-62. [Crossref] [PubMed]
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014;65:467-79. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Bookman MA. Optimal primary therapy of ovarian cancer. Ann Oncol 2016;27 Suppl 1:i58-62. [Crossref] [PubMed]
- Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26. [Crossref] [PubMed]
- Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol 2015;193:832-8. [Crossref] [PubMed]
- Nair R, Lamb BW, Geurts N, et al. The Role of Local Therapy for Oligometastatic Prostate Cancer: Should We Expect a Cure? Urol Clin North Am 2017;44:623-33. [Crossref] [PubMed]
- Sridharan S, Warde P. The importance of local control in high-risk locally advanced prostate cancer. Curr Oncol 2012;19:S6-12. [Crossref] [PubMed]
- Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 2014;65:1058-66. [Crossref] [PubMed]
- Scardino P. Update: NCCN prostate cancer Clinical Practice Guidelines. J Natl Compr Canc Netw 2005;3 Suppl 1:S29-33. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol 2014;38:435-41. [Crossref] [PubMed]
- Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol 2014;66:602-3. [Crossref] [PubMed]
- Löppenberg B, Dalela D, Karabon P, et al. The Impact of Local Treatment on Overall Survival in Patients with Metastatic Prostate Cancer on Diagnosis: A National Cancer Data Base Analysis. Eur Urol 2017;72:14-9. [Crossref] [PubMed]
- Parikh RR, Byun J, Goyal S, et al. Local Therapy Improves Overall Survival in Patients With Newly Diagnosed Metastatic Prostate Cancer. Prostate 2017;77:559-72. [Crossref] [PubMed]
- Rusthoven CG, Jones BL, Flaig TW, et al. Improved Survival With Prostate Radiation in Addition to Androgen Deprivation Therapy for Men With Newly Diagnosed Metastatic Prostate Cancer. J Clin Oncol 2016;34:2835-42. [Crossref] [PubMed]
- Satkunasivam R, Kim AE, Desai M, et al. Radical Prostatectomy or External Beam Radiation Therapy vs No Local Therapy for Survival Benefit in Metastatic Prostate Cancer: A SEER-Medicare Analysis. J Urol 2015;194:378-85. [Crossref] [PubMed]
- Shao YH, Kim S, Moore DF, et al. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. Eur Urol 2014;65:693-700. [Crossref] [PubMed]
- Sooriakumaran P, Nyberg T, Akre O, et al. Survival Among Men at High Risk of Disseminated Prostate Cancer Receiving Initial Locally Directed Radical Treatment or Initial Androgen Deprivation Therapy. Eur Urol 2017;72:345-51. [Crossref] [PubMed]
- Robinson D, Garmo H, Lissbrant IF, et al. Prostate Cancer Death After Radiotherapy or Radical Prostatectomy: A Nationwide Population-based Observational Study. Eur Urol 2018;73:502-11. [Crossref] [PubMed]
- Moschini M, Morlacco A, Kwon E, et al. Treatment of M1a/M1b prostate cancer with or without radical prostatectomy at diagnosis. Prostate Cancer Prostatic Dis 2017;20:117-21. [Crossref] [PubMed]
- Leyh-Bannurah SR, Gazdovich S, Budaus L, et al. Local Therapy Improves Survival in Metastatic Prostate Cancer. Eur Urol 2017;72:118-24. [Crossref] [PubMed]
- Shao YH, Albertsen PC, Shih W, et al. The impact of PSA testing frequency on prostate cancer incidence and treatment in older men. Prostate Cancer Prostatic Dis 2011;14:332-9. [Crossref] [PubMed]
- Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw 2014;12:686-718. [Crossref] [PubMed]
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 2004;5:219-28. [Crossref] [PubMed]
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [Crossref] [PubMed]
- Fossati N, Trinh QD, Sammon J, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol 2015;67:3-6. [Crossref] [PubMed]
- Wang Y, Qin Z, Wang Y, et al. The role of radical prostatectomy for the treatment of metastatic prostate cancer: a systematic review and meta-analysis. Biosci Rep 2018. [Crossref] [PubMed]
- Nakaoka H, Inoue I. Meta-analysis of genetic association studies: methodologies, between-study heterogeneity and winner's curse. J Hum Genet 2009;54:615-23. [Crossref] [PubMed]
- Moschini M, Soria F, Briganti A, et al. The impact of local treatment of the primary tumor site in node positive and metastatic prostate cancer patients. Prostate Cancer Prostatic Dis 2017;20:7-11. [Crossref] [PubMed]
- Carneiro A, Baccaglini W, Glina FPA, et al. Impact of local treatment on overall survival of patients with metastatic prostate cancer: systematic review and meta-analysis. Int Braz J Urol 2017;43:588-99. [Crossref] [PubMed]
- Psaila B, Lyden D. The Metastatic Niche: Adapting the Foreign Soil. Nat Rev Cancer 2009;9:285. [Crossref] [PubMed]
- Stoyanova T, Cooper AR, Drake JM, et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc Natl Acad Sci U S A 2013;110:20111-6. [Crossref] [PubMed]