
Lymphotoxin beta receptor is associated with regulation of microRNAs expression and nuclear factor-kappa B activation in lipopolysaccharides (LPS)-stimulated vascular smooth muscle cells
Introduction
Vascular inflammation and remodeling are important pathological features of atherosclerosis and vascular smooth muscle cells (VSMCs) play an important role in vascular inflammation and remodeling (1). VSMCs highly express toll-like receptor 4 (TLR4) and can be activated by lipopolysaccharides (LPS). Activated VSMCs can act like immune cells to synthesize and secrete inflammatory mediators to regulate the inflammatory responses which promote the development of vascular inflammation (1,2). And pathophysiological inflammatory processes in VSMCs are mediated by multiple molecules such as inflammatory cytokines and chemokines, adhesion molecules, transcription factors, and miRNAs (3,4).
Increased cytokines [interleukin-8 (IL-8), IL-18, monocyte chemoattractant protein (MCP-1)], adhesion molecules [intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1)] are demonstrated to play an important role in the development of atherosclerosis and their expressions are regulated by nuclear factor-kappa B (NF-κB) (5-7). NF-κB family consists of five members: p65 (or RelA), RelB, c-Rel, p50 (NF-κB1) and p52 (NF-κB2). The activated NF-κB pathway can be divided into classical and non-classical pathway. The classical NF-κB pathway is mediated by IκB-p65/p50 which regulates expression of most proinflammatory genes, including E-selectin, ICAM-1, VCAM-1 and IL-18. While the non-classical NF-κB pathway is mediated by NF-κB-inducing kinase (NIK) and IκB kinase α (IKKα)-dependent phosphorylation of p52/p100 which results in the transcription of pro-inflammatory chemokines (8,9). The best studied stimuli to induce classical NF-κB pathway are TNF and LPS, and both classical and non-classical NF-κB pathway can be induced by the activated lymphotoxin beta receptor (Ltβr) (10,11). Ltβr is a member of the tumor necrosis factor receptor superfamily and constitutively expresses on a wide variety of cells including VSMCs (12,13). Ltβr has been identified as a key mediator in multiple physiological and pathological processes by interacting with NF-κB (14). Moreover, VSMCs-Ltβr has been reported to protect against atherosclerosis by maintaining structure, cellularity and size of artery tertiary lymphoid organs in mice (13). However, how Ltβr regulates NF-κB signaling pathway in LPS-stimulated smooth muscle cells (SMCs) is not completely known.
Besides, dysregulation of microRNAs (miRNAs) expression is demonstrated to modulate inflammatory responses by interfering NF-κB signaling pathway (15) and is associated with many diseases such as atherosclerosis, cancers and diabetes (7,16). miRNAs are a class of highly-conserved, non-coding RNAs of 18-25 nucleotides in length. miRNAs interact with their targets genes and mediate indispensable and negative regulators of gene expression at the post-transcription level (17).
Therefore, in this study, we investigated whether Ltβr regulated LPS-induced inflammation in VSMCs by modulating miRNAs and NF-κB activation which might offer new targets for treatment of cardiovascular diseases, such as atherosclerosis.
Methods
Reagents and antibodies
Mouse IL-18 ELISA kit was bought from Cusabio biotech. Primary antibodies: polyclonal rabbit anti-Ltβr was from Abcam (catalog number: ab70063), rabbit anti-phosphor-NF-κB p65 (p-p65, Ser536, catalog number: 3033) and rabbit anti-p65 (catalog number: 8242) were from Cell Signaling Technology and monoclonal mouse anti-GAPDH (catalog number: HC301) TransGen biotech. Second antibodies: peroxidase-affinipure goat anti-rabbit IgG (H+L) (catalog number: 101-035-003) and peroxidase-affinipure goat anti-mouse IgG (H+L) (catalog number: 115-545-166) from Jackson ImmunoResearch. LPS were bought from Sigma.
Cell culture and treatments
Mouse aortic SMC line (MOVAS cells) was bought from ATCC. Cells were cultured in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified, 5% CO2 atmosphere. Normal MOVAS cells or shLtβr transduced MOVAS cells were treated with or without LPS (1 µg/mL) for 16 or 24 h in 5% FBS medium. Each treatment was triplicated with different passage of MOVAS cells.
Enzyme-linked immunosorbent assay (ELISA)
After confluence, cells were treated with or without LPS (1 µg/mL) for 16 or 24 h in 5% FBS medium. Then the medium was centrifuged for 15 minutes at 1,000 g, 4 °C to remove particulates. The medium was stored at −20 °C until the assay started. The level of IL-18 in medium was determined by using ELISA kit according to the manufacturer’s instruction. The concentration was calculated according to the absorbance of the samples and the standard curve.
Real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from MOVAS and shLtβr-MOVAS cells using TRIzol reagent and reversely transcribed into cDNA with GoTap® RT Master Mix according to the manufacturer’s protocol. qPCR was performed using a 20 µL reaction containing 10 µL GoTap® RT Master Mix, 1 µL 10 µm forward primer, 1 µL 10 µm reverse primer 1.5 µL template DNA and 6.5 µL nuclease-free water. The instrumentation used was the Bio-Rad, MiniOption real-time PCR machine. Initial denaturation was at 95 °C for 120 seconds followed by 40 cycles of 95 °C denaturation for 15 seconds, and 60 °C annealing and extension for 30 seconds. Positive and negative controls were tested in each run.
The specific primers used in this study were as follows: Mus-Ltβr: 5'-GCAGCCAAGACACGGTTTG-3' (forward), Mus-Ltβr: 5'-AGCCCAGCACAATGTCACAG-3' (reverse); Mus-Vcam1: 5'-CACAAGTTGGGGATTCGGTT-3' (forward), Mus-Vcam1: 5'-CCTCAAAACCCACAGAGCTCA-3' (reverse); Mus-Il18: 5'-TCCTTTGAGGAAATGGATCCAC-3' (forward), Mus-Il18: 5'-TGGCAAGCAAGAAAGTGTCCT-3' (reverse); GAPDH: 5'-GTATGACTCTACCCACGGCAAGT-3' (forward), 5'-TTCCCGTTGATGACCAGCTT-3' (Reverse).
The cycle threshold (Ct) obtained for target gene expression was normalized to GAPDH. Efficiency of reaction was calculated from the slope using the formula E = 10(−1/slope). The relative expression levels of target genes (normalized to that of GAPDH) were calculated using the 2−ΔΔCt method (18). All qPCR experiments were repeated three times.
Western blot analysis
After treatment, cells were washed twice with ice-cold PBS, lysed with RIPA lysis buffer, centrifuged and quantified with a Bradford protein assay kit according to the manufacturer’s instruction. Proteins were separated by 10% SDS-PAGE and then transferred to PVDF membranes. Membranes were blocked in 5% nonfat milk/TBST for 1 h at room temperature and then incubated with primary antibodies [polyclonal rabbit anti-Ltβr (1:1,000), rabbit anti-p-p65 (1:1,000), rabbit anti-p65 (1:1,000) and monoclonal mouse anti-GAPDH (1:5,000)] overnight at 4 °C. After three times washes, membranes were incubated with second antibodies [goat anti-rabbit IgG (H+L) (1:3,000) and goat anti-mouse IgG (H+L) (1:3,000)] for 1 h, washed three times and subsequently visualized using ECL kit.
MOVAS transduction with lentiviral short hairpin Ltβr (shLtβr) vectors
RNA interference (RNAi) is widely used in gene knockdown analysis and as a tool to investigate the function of specific genes or proteins. In order to investigate the role of Ltβr in VSMCs inflammation, the expression of Ltβr was silenced in MOVAS as previously described. The stable complex of shLtβr (interference sequence: GCCAAGACACGGTTTGCAA) and lentiviral vector (LV3-M-Ltβr-shRNA1) was constructed by GenePharma. The control group was transfected with the complex of non-targeting controls-shRNA (validated not to affect any gene) and lentiviral vector. Before transduction, 1×105 cells/well were cultured in 24-well plates for 18–24 h. When cells were about 2×105 cells/well, cells were incubated with complex and 6 µg/mL polybrene in 2 mL medium without FBS for 4 h. Then 2 mL fresh medium was added into each well for diluting the concentration of polybrene. After 12–24 h transduction, the medium was then changed into normal medium and cultured for 48 h. Seventy-two hours after transduction, the cells were stimulated with LPS as described above and then harvested for Western blotting or total RNA isolation.
Small RNA sequencing (smRNA-seq) and data analysis
smRNA-seq was performed as previously described (18). Briefly, total RNA was isolated as above mentioned. Then RNA samples were first DNase-treated and assessed for total quality using Agilent 2100 Bioanalyzer, followed by 2 rounds of polyadenylate positive (poly A+) selection and conversion to cDNA. RNA sequencing was performed on the Illumina HiSeq 2500 using the latest versions of sequencing reagents. TargetScan 7.0 was used to predict target genes.
Statistical analysis
The data were presented as the means ± SD. The measurement data were compared between the two groups with Student’s t-test. The statistical analyses were conducted with SPSS 24.0 software. P<0.05 or P<0.01 was considered statistically significant.
Results
LPS induced gene and/or protein expression of IL-18, VCAM-1, Ltβr and p-p65 in MOVAS cells
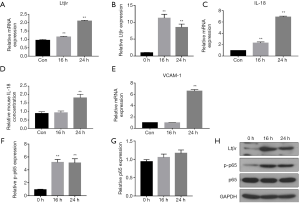
Normal MOVAS cells were first treated with or without LPS (1 µg/mL) for 0, 16 or 24 h. Then the gene and protein levels of Ltβr, IL-18, VCAM-1 and p-p65 were analyzed by qPCR, Western blot and ELISA. Ltβr, IL-18, VCAM-1 and p-p65 were expressed on unstimulated cells with relative low levels (Figure 1A,B,C,D,E,F,G). mRNA and protein expression of Ltβr were significantly (P<0.01) increased by 16 and 24 h LPS stimulation (Figure 1A,B), mRNA expression of IL-18 was increased by 16 and 24 h LPS stimulation, while protein level of IL-18 in medium was only increased by 24 h LPS stimulation (Figure 1C,D). VCAM-1 mRNA expression was only increased by 24 h LPS stimulation (Figure 1E). Protein expression of phosphorylated p65 was also significantly (P<0.01) increased by 16 and 24 h LPS treatment and non-phosphorylated p65 was not changed by LPS stimulation (Figure 1F,G,H). Therefore, 24 h stimulation was chosen for the following experiments.
LPS-induced expression of inflammatory factors was attenuated in shLtβr transduced MOVAS cells
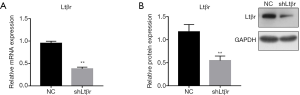
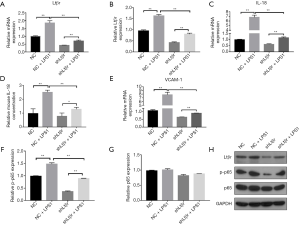
To investigate the interaction between Ltβr and LPS-induced inflammation in MOVAS cells, Ltβr was silenced by using Ltβr-specific shRNA and examined the levels of inflammatory factors including IL-18, VCAM-1 and p-p65. We first found that at 72 h after shLtβr transduction, more than 60% gene and protein expression of Ltβr was significantly and stably inhibited compared with the control group (Figure 2A,B), while cell viability was unaffected (data not shown). Then, we examined the levels of LPS-induced inflammatory factors including Ltβr, IL-18, VCAM-1 and p-p65 in shLtβr transduced MOVAS cell. mRNA and protein levels of Ltβr were still upregulated by LPS stimulation. However, the levels were significantly lower than in LPS-stimulated non-shLtβr MOVAS cells (Figure 3A,B). LPS-induced IL-18, VCAM-1 and p-p65 expression in shLtβr transduced cells showed similar trend with expression of Ltβr (Figure 3C,D,E,F,G,H).
Altered endogenous miRNA biogenesis by shLtβr transduction
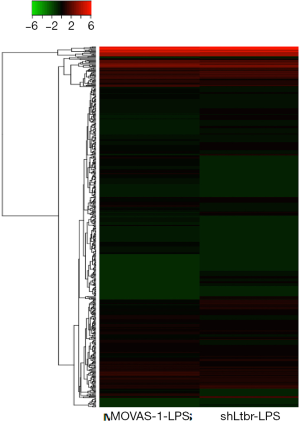
As mentioned above, miRNAs played an important role in regulating inflammation. Therefore, whether miRNAs expression was changed by shLtβr transduction was determined by smRNA-Seq analysis. LPS-induced different miRNAs expression in only lentiviral vector (without shLtβr sequence)-transduced MOVAS cells and lentiviral vector/shLtβr-transduced MOVAS cells was compared. With smRNA sequencing analysis, a total of 1,917 miRNAs were identified in the study. Based on a 95% confidence level, cutoff values of 2-fold for upregulated and downregulated genes were used to define a gene as being differently expressed gene in present study. Through global normalization of the raw data, heatmap data indicated that 10 miRNAs were upregulated and 64 miRNAs were downregulated (Figure 4). And 64 downregulated miRNAs in shLtβr transduced MOVAS cells were listed in Table 1.
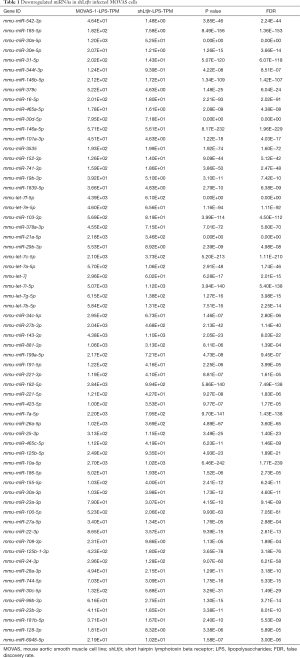
Full table
miR-146b-5p and miR-27a-5p levels were significantly decreased in shLtβr transduced MOVAS cells
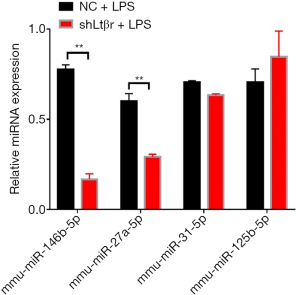
For further study, miRNAs were chosen based on (I) decreased miRNAs expression in LPS-stimulated shLtβr MOVAS cells compared with LPS-stimulated normal cells; (II) upregulated miRNAs expression in inflammation found in previous study. Then the expression level of selected miRNAs in samples was confirmed by qPCR. And results showed that relative miR-146b-5p and miR-27a-5p expression was significantly downregulated in LPS-stimulated shLtβr MOVAS cells compared with LPS-stimulated normal MOVAS cells (Figure 5).
Discussion
VSMCs inflammation induced by LPS characterized by increased production of inflammatory cytokines, chemokines, adhesion molecules, transcription factors and changed miRNAs expression which were involved in the development of atherosclerosis and connect reciprocally (3). Therefore, in present report, we conducted experiments to reveal the downstream of Ltβr and its regulatory effects on miRNAs/NF-κB signaling pathway and subsequent inflammatory cytokine and adhesion molecule expression in LPS-stimulated VSMCs for the first time.
Firstly, Ltβr was found to be expressed on non-stimulated VSMCs and increased by LPS stimulation which suggested its role in regulation of inflammation in VSMCs. In previous study, Ltβr activation was involved in inflammatory responses which was mediated by activating classical and non-classical NF-κB pathway (14,19). NF-κB is a ubiquitous transcription factor and NF-κB activation is reported to be crucial for the expression of inflammatory mediators including adhesion molecules (VCAM-1) and cytokines (IL-18) in VSMCs (20-22). VCAM-1 expresses not only on endothelial cells but also SMCs and facilitates the accumulation of transmigrated immune cells within the vascular walls in the development of atherosclerosis (23). IL-18 is a pro-atherogenic cytokine which is highly expressed in SMCs of atherosclerotic lesions. Recombinant IL-18 increases cytokines production and intensify adhesion molecules expression in endothelial cells (6). Lower expression of IL-18 was accompanied by less development of atherosclerosis in mice (20). Furthermore, elevated levels of plasma IL-18 are associated with the extent of coronary atherosclerosis (24). Therefore, downregulation of VCAM-1 and IL-18 in inflammation will be beneficial for atherosclerosis. In present study, LPS-increased VCAM-1 and IL-18 were restored by shLtβr transduction. Furthermore, LPS stimulation-induced the phosphorylation and subsequent translocation of p65 to the nucleus in normal MOVAS cells were also inhibited by shLtβr transduction. All these findings indicated that Ltβr-regulated VSMCs inflammation including IL-18 production and VCAM-1 expression might be associated with its role in modulating the activation of NF-κB. As we mentioned above, miRNAs were reported to play an important role in regulating the inflammatory responses by modulating gene expression of upstream factors of NF-κB signaling pathway or directly modulating gene expression of subunits of NF-κB (25,26). Dysregulation of miRNAs was associated with the development of vascular inflammation (27). Therefore, whether miRNAs expression was influenced by shLtβr transduction has also been investigated. miRNAs expression was dysregulated and miR-146b-5p and miR-27a-5b were significantly downregulated in LPS-treated shLtβr cells which were accompanied with inhibited phosphorylation of p65 and NF-κB-mediated inflammatory factors, such as IL-18 and VCAM-1.miR-146b-5p acted as an inhibitor of NF-κB-mediated inflammation by targeted expression of IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6), upstream regulators of NF-κB, in monocytes and endothelial cells. Increased expression of IRAK1 and TRAF6 leaded to more NF-κB p65 DNA binding activity. Altered p65 nuclear translocation leaded to changes in the expression of downstream targets of NF-κB signaling including VCAM-1 expression (25,28). Besides, miRNAs were also reported to directly regulate NF-κB subunit by silencing NFκB1 and p65 to inactivate canonical NF-κB signaling (29). miR-146b-5p was found to target NFκB1 gene (data not shown).
Inhibition of miR-27a expression significantly downregulated the expression of TNFα and IL-6 which was associated with altering the expression of both repressors and activators of NF-κB signaling cascade including RelA gene leading to decreased p65 nuclear translocation in response to proinflammatory stimulant which suggests miR-27a-5p act to regulate the extent of NF-κB signaling (26,30).
Conclusions
In summary, two new findings were demonstrated in present study: (I) LPS-induced NF-κB activation and subsequent pro-inflammatory factors (IL-18 and VCAM-1) expression were inhibited by silencing Ltβr on MOVAs cells; (II) the expression levels of miR-146b-5p and miR-27a-5p which were reported to modulate the NF-κB activation were downregulated by shLtβr transduction. We made a conclusion that Ltβr could regulate VSMCs inflammation in atherosclerosis by modulating miRNAs/NF-κB/IL-18-VCAM-1 cascade. These findings verify the role of Ltβr in VSMCs inflammation and provide a potential signaling cascade mediated the effects of Ltβr which offers new insights into the molecular mechanisms underlying VSMCs dysregulation and pathogenesis of atherosclerosis.
Acknowledgments
Funding: This work was supported by the President Foundation of Nanfang Hospital, Southern Medical University (grant No. 2017B022). National Natural Science Foundation of China (grant No. 81600321). Science and Technology program of Guangzhou (grant No. 201804010067).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.03.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 2015;214:33-50. [Crossref] [PubMed]
- Wang P, Han X, Mo B, et al. LPS enhances TLR4 expression and IFNgamma production via the TLR4/IRAK/NFkappaB signaling pathway in rat pulmonary arterial smooth muscle cells. Mol Med Rep 2017;16:3111-6. [Crossref] [PubMed]
- Varghese JF, Patel R, Yadav UCS. Novel Insights in the Metabolic Syndrome-induced Oxidative Stress and Inflammation-mediated Atherosclerosis. Curr Cardiol Rev 2018;14:4-14. [Crossref] [PubMed]
- Braun M, Pietsch P, Schrör K, et al. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res 1999;41:395-401. [Crossref] [PubMed]
- Li M, Van Esch BCAM, Henricks PAJ, et al. Time and Concentration Dependent Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Induced Endothelial Activation. Front Pharmacol 2018;9:233. [Crossref] [PubMed]
- Esfahani M, Saidijam M, Najafi R, et al. The effect of salusin-β on expression of pro- and anti-inflammatory cytokines in human umbilical vein endothelial cells (HUVECs). ARYA Atheroscler 2018;14:1-10. [PubMed]
- Laffont B, Rayner KJ. MicroRNAs in the Pathobiology and Therapy of Atherosclerosis. Can J Cardiol 2017;33:313-24. [Crossref] [PubMed]
- Mussbacher M, Salzmann M, Brostjan C, et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front Immunol 2019;10:85. [Crossref] [PubMed]
- Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009;1:a000034. [Crossref] [PubMed]
- Madge LA, Kluger MS, Orange JS, et al. Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells. J Immunol 2008;180:3467-77. [Crossref] [PubMed]
- Piao W, Xiong Y, Famulski K, et al. Regulation of T cell afferent lymphatic migration by targeting LTβR-mediated non-classical NFκB signaling. Nat Commun 2018;9:3020. [Crossref] [PubMed]
- Lan J, Heneghan AF, Sano Y, et al. Parenteral nutrition impairs lymphotoxin β receptor signaling via NF-κB. Ann Surg 2011;253:996-1003. [Crossref] [PubMed]
- Hu D, Mohanta SK, Yin C, et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors. Immunity 2015;42:1100-15. [Crossref] [PubMed]
- Fernandes MT, Dejardin E, dos Santos NR. Context-dependent roles for lymphotoxin-beta receptor signaling in cancer development. Biochim Biophys Acta 2016;1865:204-19. [PubMed]
- Yang Y, Wang JK. The functional analysis of MicroRNAs involved in NF-kappaB signaling. Eur Rev Med Pharmacol Sci 2016;20:1764-74. [PubMed]
- Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol Cancer 2014;13:33. [Crossref] [PubMed]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 2008;9:102-14. [Crossref] [PubMed]
- Pacheco NL, Heaven MR, Holt LM, et al. RNA sequencing and proteomics approaches reveal novel deficits in the cortex of Mecp2-deficient mice, a model for Rett syndrome. Mol Autism 2017;8:56. [Crossref] [PubMed]
- Dai X, Thiagarajan D, Fang J, et al. SM22α suppresses cytokine-induced inflammation and the transcription of NF-κB inducing kinase (Nik) by modulating SRF transcriptional activity in vascular smooth muscle cells. PLoS One 2017;12:e0190191. [Crossref] [PubMed]
- Stein S, Lohmann C, Handschin C, et al. ApoE-/- PGC-1α-/- mice display reduced IL-18 levels and do not develop enhanced atherosclerosis. PLoS One 2010;5:e13539. [Crossref] [PubMed]
- Kim HJ, Park KG, Yoo EK, et al. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal 2007;9:301-7. [Crossref] [PubMed]
- Li P, Sanz I, O'Keefe RJ, et al. NF-kappa B regulates VCAM-1 expression on fibroblast-like synoviocytes. J Immunol 2000;164:5990-7. [Crossref] [PubMed]
- Kasper HU, Schmidt A, Roessner A. Expression of the adhesion molecules ICAM, VCAM, and ELAM in the arteriosclerotic plaque. Gen Diagn Pathol 1996;141:289-94. [PubMed]
- Mysliwska J, Wypych J, Suchanek H. Re: Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6 Atherosclerosis 2007;195:415-6. [Atherosclerosis 188 (2006) 450–454]. [Crossref] [PubMed]
- Hulsmans M, Van Dooren E, Mathieu C, et al. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS One 2012;7:e32794. [Crossref] [PubMed]
- Romay MC, Che N, Becker SN, et al. Regulation of NF-κB signaling by oxidized glycerophospholipid and IL-1β induced miRs-21-3p and -27a-5p in human aortic endothelial cells. J Lipid Res 2015;56:38-50. [Crossref] [PubMed]
- Ding Y, Sun X, Shan PF. MicroRNAs and Cardiovascular Disease in Diabetes Mellitus. Biomed Res Int 2017;2017:4080364. [Crossref] [PubMed]
- Echavarria R, Mayaki D, Neel JC, et al. Angiopoietin-1 inhibits toll-like receptor 4 signalling in cultured endothelial cells: role of miR-146b-5p. Cardiovasc Res 2015;106:465-77. [Crossref] [PubMed]
- Huang T, Kang W, Zhang B, et al. miR-508-3p concordantly silences NFKB1 and RELA to inactivate canonical NF-κB signaling in gastric carcinogenesis. Mol Cancer 2016;15:9. [Crossref] [PubMed]
- Wang Z, Ruan Z, Mao Y, et al. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol 2014;290:190-5. [Crossref] [PubMed]


