
Impact of blood-brain barrier disruption on newly diagnosed neuromyelitis optica spectrum disorder symptoms and prognosis
Introduction
Aquaporin-4 antibody (AQP4-IgG) is a specific NMOSD marker and the discovery of AQP4-IgG in NMOSD patients is the first clue for pathogenesis of disease. Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune-mediated inflammatory demyelinating disease with relapsing-remitting tendency. Notably, AQP4-IgG may persist in serum for many years, even with the absence of symptoms (1); therefore, the pathogenesis of NMOSD is still not fully understood.
Adding onto this, It is currently believed that the central pathogenic event in NMOSD is the entry of AQP4-IgG into the CNS through a damaged blood-brain barrier (BBB), triggering complement activation and granulocyte infiltration leading to astrocyte death (2). At the cellular level, AQP4 is expressed in astrocyte foot processes, with particularly abundant expression in the optic nerve, spinal cord, brainstem, hypothalamus, and cerebral ventricles, where it serves to maintain water balance by regulating the circulation of intracellular and extracellular water (3).
In AQP4-IgG seropositive rats, disruption of the BBB using pulsed ultrasound-induced NMO-like lesions in the brain and spinal cord by allowing entry of serum IgG. Central nervous system (CNS) inflammation, demyelination, and astrocyte damage are the primary pathological manifestations of NMO (4), and aberrant BBB permeability is associated with greater Extended Dysfunction Status Scale (EDSS) score and spinal cord lesion length (5). Moreover, BBB permeability, as assessed by the qalb, is strongly related to the degree of disability and brain atrophy after MS onset (6). In NMO spectrum disorder (NMOSD) as well, albumin quotient (qalb) is associated with the degree of motor disability (7).
However, there have been few studies on NMOSD in the acute phase, so there is a dearth of clinical and prognostic data on this patient population. Given the importance of BBB permeability in NMOSD pathogenesis, a convenient and straightforward biomarker is needed to evaluate disease severity and treatment outcome, predict recurrence, and guide individualized treatment decisions in newly diagnosed NMOSD patients. Therefore, we compared multiple peripheral types of blood and cerebrospinal fluid (CSF) metrics, magnetic resonance imaging (MRI) signs, and EDSS scores between NMOSD patients with high or normal BBB permeability as assessed by the qalb. The primary aim of this investigation is to determine whether higher BBB permeability during the acute phase of NMOSD is associated with greater symptom severity and lower post-treatment change.
Methods
Ethics statements
The Ethics Committee approved the study of the First Affiliated Hospital of Guangxi Medical University.
Study population
We included 46 NMOSD patients admitted to the Department of Neurology, First Affiliated Hospital of Guangxi Medical University from January 2017 to March 2019. The cohort included 4 males and 42 females ranging from 14 to 78 years old. Age at onset was 42.9±15.5 years, and the average disease duration was 1.14±1.05 months. Symptoms gradually increased following onset. All patients were diagnosed based on the 2015 NMOSD diagnostic criteria developed by the International Panel for NMO Diagnosis (IPND). All patients provided informed consent to blood and CSF sampling by venipuncture and lumbar puncture, respectively. Exclusion criteria were treatment with methylprednisolone, gamma globulin, or plasma replacement before hospitalization, or diseases such as myasthenia gravis, meningitis, brain abscess, and epilepsy that may affect qalb index and EDSS score. In this study, BBB permeability was assessed according to qalb = (4 + age/15) × 10−3, increased BBB permeability was defined as qalb > (4 + Age/15) × 10−3 (8). And all tests and qalb index calculations were performed before treatment.
Treatment
All patients with newly diagnosed NMOSD were treated with methylprednisolone 1.0 g/day for 3 days.
Expanded disability status scale score
The EDSS score was used to assess the severity of symptoms before and after treatment. An experienced neurologist performed the entire evaluation procedure.
MRI images
All patients were examined by T1-weighted, T2-weighted, and T2-Flair imaging of the optic nerve, brain, cervical spinal cord, and thoracic spinal cord using a 1.5-T MRI system. An experienced neuroradiologist reviewed the images.
AQP4-IgG analysis
According to the latest guidelines, AQP4-IgG was detected by indirect immunofluorescence based on cell transfection.
Peripheral blood (PB) and CSF analysis
Total leukocyte, neutrophil, CD4+ T cell, CD8+ T cell, and total T lymphocyte counts, as well as albumin, complement C3, complement C4, and AQP4-IgG titer, autoimmune antibody (ANA/SSA/SSB/Ro-52) concentrations, were measured from serum samples. In addition, total leukocyte count and total protein, albumin, and AQP4-IgG concentrations were measured from CSF samples. Measures of CD4+ T cells, CD8+ T cells, complement C3, and C4, and ANA/SSA/SSB/Ro-52 were not all conducted in nine patients, but all other measures were included in the analysis.
Statistical analysis
All statistical analyses were performed using SPSS 24.0 software. Categorical data are expressed as ratios and compared between groups by the chi-square test. Continuous data with normal distributions are expressed as
Results
Clinical features
Patients were divided into high and normal BBB permeability groups according to the qalb, with 15/46 patients (33%) in the qalb increased group [qalb > (4 + age/15) × 10−3] and the remainder in the qalb normal group [qalb ≤ (4 + age/15) × 10−3] (Table 1).
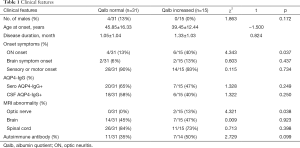
Full table
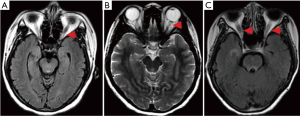
Increased BBB permeability increased the probability of optic neuritis
The probability of patients with optic neuritis was significantly higher in the qalb increased group (P=0.037). Similarly, the probability of patients with abnormal optic nerve MR imaging manifestations on MR images was higher in the qalb increased group (P=0.038) (Figure 1).
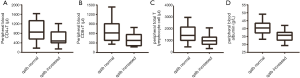
BBB permeability and PB abnormalities
Compared to the qalb normal group, the qalb increased group demonstrated no significant differences in total leukocyte count (P=0.200), neutrophil count (P=0.761), total lymphocyte count (P=0.679), complement C3 concentration (P=0.705), and complement C4 concentration (P=0.306). However, the qalb increased group exhibited significantly lower serum albumin (P=0.001) and CD4+ T cell count (P=0.044), CD8+ T cell count (P=0.014), and total T lymphocyte count (P=0.016) (Figure 2).
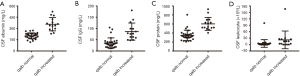
BBB permeability and CSF abnormalities
The total number of leukocytes in the CSF was significantly elevated in the qalb increased group (P=0.007). Similarly, the qalb increased group demonstrated elevated CSF albumin, IgG, and total protein (all P=0.000) (Figure 3).
BBB permeability was associated with poorer treatment response
Patients in the qalb normal group showed better symptom relief following methylprednisolone treatment than the qalb increased group, as indicated by the change in the EDSS score (P=0.028) (Table 2).

Full table
Discussion
While several previous studies have explored the relationship between qalb and NMOSD’s clinical characteristics, it is unclear the effect of qalb on newly diagnosed NMOSD. We found that BBB disruption, as evidenced by high qalb, is associated with optic nerve abnormalities on MR images, poorer treatment response, lower CD4+ and CD8+ T cells in peripheral blood, and elevated total protein and leukocytes in CSF. Collectively, these blood and CSF changes are consistent with increased BBB permeability as a significant factor influencing NMOSD severity and treatment response in the acute phase.
Characteristics of the CNS microvasculature confer the BBB, and under normal circumstances, provides a continuous barrier against peripheral cells (such as leukocytes), proteins, and other factors. Furthermore, BBB component cells express multiple transport pathways that can regulate the exchange of materials between the PB and the CNS. This selective barrier function is critical for maintaining the optimal extracellular microenvironment for neuronal signaling in the CNS. The BBB also protects the CNS from peripheral inflammatory factors and cells as well as viruses, bacteria, and bacterial toxins. Disruption of BBB integrity allows these factors, including immunocytes and pro-inflammatory cytokines, to access vulnerable neuronal populations, ultimately inducing neuroinflammatory diseases (9). Astrocytes are a critical component of the BBB and regulate barrier function (10). In addition, astrocytes couple neuronal activity to local blood flow thus mediating homeostatic regulation of neuronal metabolism (11). Astrocytes also promote angiogenesis by secreting angiopoietin 1 (Ang 1) and altering BBB permeability by regulating junctional protein expression (12). Aquaporin 4 (AQP4) is highly expressed in astrocyte foot processes that abut the neurovasculature, with particularly high expression in the brainstem, optic nerve, spinal cord, and periventricular regions (13), where it functions mainly to maintain water homeostasis. These regions of high AQP4 expressional are also the sites of typical NMO lesions (14), strongly implicating AQP4 in disease pathogenesis. Indeed, it is believed that under BBB degradation, AQP4-IgG invades the CNS and binds to AQP4 on astrocytes, triggering an immune cascade that ultimately leads to astrocyte death, loss of extracellular and metabolic homeostasis, and further BBB degradation (13).
However, nearly one-third of NMO patients are seronegative for AQP4-IgG (14). Therefore, AQP4-IgG titer provides no prognostic information in these cases. Alternatively, our results suggest that qalb can be used for the prognosis of all NMOSD patients. We report that BBB disruption leads to worse prognosis in NMOSD, by allowing pro-inflammatory factors, leukocytes, and AQP4 antibodies to invade the retinal or CNS parenchyma, resulting in neuroinflammation. Our findings are in accord with previous reports that qalb is associated with CSF albumin, CSF leukocyte count, and spinal cord lesion length (5). In addition, patients with increased qalb demonstrated a higher probability of developing optic neuritis, a finding not previously reported. Retinal nerve fiber layer (RNFL) loss is more severe in NMO than in MS according to optical coherence tomography (OCT) (15). The onset of optic neuritis in NMOSD is believed to result from the entry of T cells into the CNS through the disrupted BBB. During optic neuritis, the retinal nerve fiber/ganglion cell layer often becomes thinner (16), suggesting damage to the optic nerve and retina (17). T cells can enter the retina through two pathways, the vascular pathway of the nerve fiber/ganglion cell/inner plexus layer or the outer nucleus/outer plexus layer/core layer, AQP4-IgG, and complement resulting in the loss of AQP4 and destruction of Müller glial cells (18). This phenomenon has also been described in the autopsy results of NMOSD patients (19).
The mechanism of CD4+ T and CD8+ T cell reduction in PB of NMO is complex. OX40 (CD134), a member of the tumor necrosis factor (TNF) receptor family expressed primarily on activated CD4+ T and CD8+ T cells, was found to be downregulated in PB of NMO patients (20). Further, both the current results and a previous study (21) have found lower ratio PB CD4+ T cells and CD8+T cells, suggesting that BBB disruption is associated with a peripheral blood lymphocyte (PBL) imbalance. Based on earlier studies, we speculate that this reduction is related to the disruption of immune homeostasis. Linhares et al. (22) reported enhanced cell death in PHA-activated cultures from NMO patients and low release of interleukin-2 (IL-2) and interferon-gamma (INF-γ). This lowered release of IL-2 and IFN-γ may trigger activation-induced cell death (AICD) of lymphocytes (23). Another potential mechanism is cell differentiation. Both T-helper cell 17 (Th17) and the cytokine IL-17A are reported to be abnormally increased in the PB of NMO patients. The resulting elevation in transforming growth factor-β, IL-6, and IL-21 in PB promotes the differentiation of CD4+ T cells and the secretion of cytokines. Abnormally high Th17 cell numbers elevated IL-17 secreted by CD8+ T cells, and higher IL-17A are all detected in NMO (24). In addition, Th17 cell number and IL-17A concentration are correlated with the EDSS score (25). However, we were unable to directly study this potential pathway due to an inability to detect CD4+ T cell subsets and related cytokines. Future studies are planned to assess this mechanism. Leukocytes were also significantly elevated in the CSF of patients with newly diagnosed NMOSD, especially the increased qalb group, in accord to a previous study by Wang et al. (5). We suspect that this phenomenon is related to cell migration.
Thus, the destruction of the BBB in NMOSD results in the immune invasion, which triggers the release of pro-inflammatory cytokines and complement factors from astrocytes resulting in further leukocytes infiltration (especially eosinophils and neutrophils) during the period of disease deterioration (14). This study also proves the utility of qalb as a biomarker for evaluating BBB permeability and for the prognosis of newly diagnosed NMOSD. Detection of high qalb may help guide individualized treatment.
There are limitations to this study. All patients have undergone only one lumbar puncture and only one qalb has been measured, while the effects of BBB permeability on NMOSD pathogenesis need to be confirmed by multiple lumbar punctures performed at different stages.
Acknowledgments
We want to thank Professor Yulan Tang and Shangling Pan for helpful guidance and the participating patients.
Funding: Guangxi Health Committee of China (S2019093). Guangxi Natural Science Foundation Grant (0991009, 2012GXNSFAA053082). National Natural Science Foundation of China Grant (81460194).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form and declare: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee approved the study of the First Affiliated Hospital of Guangxi Medical University [No. 2019(KY-E-119)].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bradl M, Reindl M, Lassmann H. Mechanisms for lesion localization in neuromyelitis optica spectrum disorders. Curr Opin Neurol 2018;31:325-33. [Crossref] [PubMed]
- Vincent T, Saikali P, R, Roth A, et al. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J Immunol 2008;181:5730-7. [Crossref] [PubMed]
- Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535-44. [Crossref] [PubMed]
- Yao X, Adams MS, Jones PD, et al. Noninvasive, Targeted Creation of Neuromyelitis Optica Pathology in AQP4-IgG Seropositive Rats by Pulsed Focused Ultrasound. J Neuropathol Exp Neurol 2019;78:47-56. [Crossref] [PubMed]
- Wang Y, Zhu M, Liu C, et al. Blood Brain Barrier Permeability Could Be a Biomarker to Predict Severity of Neuromyelitis Optica Spectrum Disorders: A Retrospective Analysis. Front Neurol 2018;9:648. [Crossref] [PubMed]
- Uher T, Horakova D, Tyblova M, et al. Increased albumin quotient (QAlb) in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. Mult Scler 2016;22:770-81. [Crossref] [PubMed]
- Tomizawa Y, Yokoyama K, Saiki S, et al. Blood-brain barrier disruption is more severe in neuromyelitis optica than in multiple sclerosis and correlates with clinical disability. J Int Med Res 2012;40:1483-91. [Crossref] [PubMed]
- Reiber H, Otto M, Trendelenburg C, et al. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin Chem Lab Med 2001;39:324-32. [Crossref] [PubMed]
- Daneman R, Prat A. The Blood–Brain Barrier. Cold Spring Harbor Perspectives in Biology 2015;7:a020412. [Crossref] [PubMed]
- Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 1987;325:253-7. [Crossref] [PubMed]
- Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010;468:232-43. [Crossref] [PubMed]
- Prat A, Biernacki K, Wosik K, et al. Glial cell influence on the human blood-brain barrier. Glia 2001;36:145-55. [Crossref] [PubMed]
- Wingerchuk DM. Neuromyelitis optica spectrum disorders. Continuum (Minneap Minn) 2010;16:105-21. [Crossref] [PubMed]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805-15. [Crossref] [PubMed]
- Levin MH, Bennett JL, Verkman AS. Optic neuritis in neuromyelitis optica. Prog Retin Eye Res 2013;36:159-71. [Crossref] [PubMed]
- Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler 2015;21:678-88. [Crossref] [PubMed]
- Zeka B, Hastermann M, Kaufmann N, et al. Aquaporin 4-specific T cells and NMO-IgG cause primary retinal damage in experimental NMO/SD. Acta Neuropathol Commun 2016;4:82. [Crossref] [PubMed]
- Zeka B, Lassmann H, Bradl M. Muller cells and retinal axons can be primary targets in experimental neuromyelitis optica spectrum disorder. Clin Exp Neuroimmunol 2017;8:3-7. [Crossref] [PubMed]
- Hokari M, Yokoseki A, Arakawa M, et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol 2016;79:605-24. [Crossref] [PubMed]
- Alidadiani P, Eskandari N, Shaygannejad V, et al. Expression of OX40 Gene and its Serum Levels in Neuromyelitis Optica Patients. Biomol Concepts 2019;10:62-7. [Crossref] [PubMed]
- Uzawa A, Mori M, Hayakawa S, et al. Expression of chemokine receptors on peripheral blood lymphocytes in multiple sclerosis and neuromyelitis optica. BMC Neurol 2010;10:113. [Crossref] [PubMed]
- Linhares UC, Schiavoni PB, Barros PO, et al. The ex vivo production of IL-6 and IL-21 by CD4+ T cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol 2013;33:179-89. [Crossref] [PubMed]
- Akkoc T, de Koning PJ, Ruckert B, et al. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol 2008;121:652-8.e1. [Crossref] [PubMed]
- Wang HH, Dai YQ, Qiu W, et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 2011;18:1313-7. [Crossref] [PubMed]
- Li Y, Wang H, Long Y, et al. Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol 2011;234:155-60. [Crossref] [PubMed]


