
Imaging manifestations of bladder paraganglioma
Introduction
Bladder paraganglioma (BPG), also referred to as extra-adrenal pheochromocytoma, is a neuroendocrine neoplasm that originates from the chromaffin tissue of the sympathetic nervous system embedded in the muscle layer of the bladder wall, accounting for 0.06% of all bladder tumors and 1% of overall parangangliomas (1-3). It is an extremely rare but severe condition that may cause severe hypertensive crisis during handling and mobilization of the tumor (4). 35% of BPGs are malignant (5). However, almost one third of BPG patients do not present with tumor specific symptomatology. Tumors are mostly located in the submucosa and myometrium and are potentially malignant and easily misdiagnosed as bladder cancer (6). The previous literature is mostly based on case reports. In this study, we studied 10 cases in order to provide support the diagnosis and treatment of BPGs.
Methods
General information
The clinical and imaging data of 10 cases of BPGs confirmed by histopathological examination from June 2009 to May 2019 at Zhongshan Hospital of Fudan University were collected. Institutional review board approval with consent waiver was obtained for this retrospective study. 7 males and 3 females were enrolled, with an average age of 52.4 years (range, 16–79 years). Clinical manifestations: 4 cases of hematuria (3 cases of painless hematuria, 1 case with dysuria), 3 cases of hypertension (1 case with palpitations and palpitations after urination), 3 cases found by physical examination. All 10 cases were surgically resected. Postoperative pathology and immunohistochemistry confirmed the presence of BPG. Among them, 1 case had obvious abnormal cells, infiltrating the mucosal layer, and tumor thrombi were seen in the vessel. 4 cases were not followed up, and 6 cases had no recurrence and metastasis after 5 months to 3 years’ follow-up.
Imaging examination methods
Computed tomography (CT) examination
Siemens Somatom 64-row dual-source CT and TOSHIBA Aquilion One 320-row spiral CT were used, and 500–800 mL of water was consumed half an hour before the examination. Scanning parameters: 120 Kv, 250 mA, layer thickness 5 mm, 1 mm reconstruction. Flat scan was used, in intravenous phase (scan at 80 s after contrast injection) and renal pelvis phase (scan at 15–30 min after contrast injection), nonionic contrast agent Onipak was used, dose 1.5–2.0 mL/kg, injection rate 3 mL/s.
Magnetic resonance imaging (MRI) examination
Siemens Aera MR Scanner was used for T1-weighted images (T1WI) axial position, T2-weighted images (T2WI) sagittal and axial plain scanning and diffusion weighted imaging (DWI) sequence axial scanning. T1WI and T2WI used SE sequence. DWI used single-shot SE-EPI sequence plus fat suppression technology, and dynamic enhancement uses VIBE sequence. Scan parameters: TR 5500 ms, TE 82 ms, layer thickness 5 mm, field of view 400×400 mm, matrix 128×128, and diffusion coefficient b value selected 0, 1,000 s/mm2.
Ultrasound examination
Philips EPIQ7-III and HITACHI EUB-8500 ultrasound diagnostic instrument were used. Probe frequency was set at 2.0–5.0 MHz. Patients were instructed to moderately fill the bladder, fully expose the lower abdomen to the pubic symphysis, and the longitudinally, laterally, and in multiple directions in the bladder area were scanned by the probes.
Results
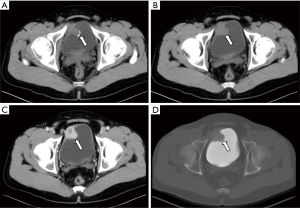
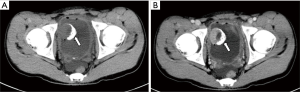
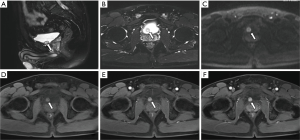
All ten neoplastic lesions were single. Among them, 5 tumor lesions were located in the right wall of the bladder (1 in the right wall, 1 in the right anterior wall, 3 in the right posterior wall), and 3 were in the left wall (1 in the left wall and 2 in the left Back wall), and 2 cases were located on the bottom wall. Nine lesions were round in shape (of which 7 cases grew inside the cavity and 2 cases grew inside and outside the cavity), and one case had irregular shapes and grew inside and outside the cavity (Figure 1). The borders of 10 cases were clear, with a length of 1–3.5 cm. Two cases of necrosis were found within the lesion (Figure 2). One case showed cystic characteristics within the solid tissue (Figure 3). Two cases were accompanied with marginal arc and semicircular calcification (Figures 1 and 2). Post-contrast CT exam showed markedly enhancement of the pathological tissue. The CT value was increased by 30–85 Hu, with an average increase of 60.1 Hu. MRI showed tumor T1WI equivalent signal, T2WI slightly higher signal accompanied by small capsule-like high signal, and DWI high signal, and the enhancement was obviously sustained (Figure 3). These data were similar to previous study, which showed that all tumors exhibited slight hyperintensity on T1WI and hyperintensity with "salt and pepper" appearance on T2WI. DWI indicated strong hyperintensity, and all cases exhibited conspicuous enhancement after intravenous Gd-DTPA injection (7). Of the 9 cases subjected to ultrasound examinations, 5 showed hypoechoic masses, and 3 were moderately echogenic masses, and 1 was hyperechoic masses. Color doppler flow imaging (CDFI) demonstrated that 5 cases showed rich color blood flow or spot-shaped, line-shaped color blood flow, 2 cases had no clear blood flow in CDFI, and 2 cases did not describe blood flow.
Discussion
BPG is a rare tumor derived from the medullary tissue of the bladder wall sympathetic nervous system, which mostly occurs in the submucosa and muscularis mucosa (8). The majority of BPGs are benign, and the only absolute criterion for malignancy is the presence of metastases at sites where chromaffin tissue is not usually found (9). BPGs could be divided into functional and non-functional according to catecholamine content and active degree of release (8). The clinical manifestations of non-functional paraganglioma are mainly painless hematuria or urinary tract obstruction. Functional paraganglioma can develop palpitations, dizziness, high blood pressure, and elevated urinary blood pressure (10). In addition, the majority of non-functional BPGs were positive for chromogranin A and Synaptophysin, but were negative for cytokeratins (11), which may be the most appropriate way to diagnose nonfunctional BGPs. Seven patients in this group were non-functional and 3 patients were functional BPG in this study. Paraganglioma of the bladder can occur anywhere in the bladder wall, mostly single. The 10 cases in this group were all single, with no specific location. Foreign literature reported that the average age of BPG was 43.3 years (11–84 years), and the male to female ratio was 1.07:1 (12). The 22 cases of BPG reported by Zhai et al. in China had an average age of 49.8 years (16–76 years), and the ratio of male to female was 1:1 (1). The average age of this group was 52.4 years (16–79 years), and the male to female ratio was 2.33:1, which was not consistent with the previous literature reported, but the sample size of current study and previous study is small, so it is not clear whether there is a correlation between gender and age and tumor occurrence.
CT and MRI manifestations
Most single-shot round soft tissue density or slightly lower density were found in BPG by CT plain scan, with uniform or uneven density and clear boundaries. MRI showed low signals for T1WI in tumors, slightly higher signals for T2WI, lower ADC signals with high signals in DWI, and enhanced scan was uniformly or unevenly enhanced. Post-contrast CT exam showed markedly enhancement of the pathological tissue, and the obvious enhancement of the lesions was helpful for the diagnosis of BPG. It reflects the characteristics of rich blood supply of BPG, which is consistent with the pathological characteristics of tumors composed of main cells and support cells and rich blood sinuses to form a vascular network (13-15). The literature reported that BPG was mostly a single submucosal and muscular layer mass growing into the bladder cavity (1). Of the 10 cases in this group, 7 cases grew inside the cavity, and 3 cases grew inside and outside the cavity, which is basically consistent with previous study, indicating that most of the BPG is closer to the submucosa, which is easy to grow into the cavity. Paraganglioma is susceptible to degeneration, so tumors show bleeding, necrosis, cystic changes, and calcification. Of the 10 patients in this group, 2 cases had lesions within the lesion and 1 case had lesions within the lesion. Asbury et al. believed that circular calcification of tumor margins was helpful for the diagnosis of BPG (14,16). Only 2 of the 10 cases in this group have marginal arcs and semi-annular calcifications. Therefore, whether ring calcification has diagnostic value needs larger sample analysis. Previous literature reported that if BPG appeared liquefied necrosis, unclear borders, and metastases, it may indicate malignancy (1). In this group, only one tumor with necrosis and calcification growing inside and outside the cavity was confirmed to be potentially malignant by pathology (no follow-up after 5 months of follow-up). The rest of the patients with necrosis, cystic changes, and calcification did not find malignancy after surgery. Therefore, the presence or absence of necrosis, cystic changes, and calcification cannot be used as a criterion for judging the benign and malignant nature, nor can the benign or malignant nature be judged solely by the abnormality of the tumor cells (1). Direct invasion of nearby organs and structures and the appearance of lymph nodes or distant organ metastases are the reliable criteria for judging the malignancy.
Ultrasound manifestations
Ultrasound examination mainly manifested as round low-echo mass with clear boundaries, and some internal echoes were uneven. CDFI showed speckled and striped blood flow signals within the mass (9). Previous study showed that BPGs were solitary, and 40% were located on the dome. The longest diameters in 80% of the BPGs were in the range 1.1–3.0 cm, and 40% of the BPGs were highly vascular (17). Of the 9 cases of ultrasonography in this group, 8 cases showed low echo and medium echo mass, and 5 cases of CDFI showed rich colored blood flow or dot-shaped, linear colored blood flow, which basically accorded with previous literatures. No clear blood flow was found in 2 cases of CDFI, which may be caused by necrosis, cystic changes, or calcification.
Differential diagnosis
BPG needs to be distinguished from other tumors of the bladder: (I) Leiomyoma is the most common non-epithelial benign tumor of the bladder, accounting for about 35% of benign tumors of the bladder and 0.5% of all tumors of the bladder (18,19). Leiomyoma is more common in young and middle-aged women. The tumoral lesions is with uniform density and signals, and the boundaries are clear. The enhancement scan displays mild to moderate strengthening. (II) bladder cancer (urinary epithelial cancer) is the most common malignant tumor of the bladder. It is more common in the elderly and is often located in the triangular area. The bladder wall is locally thickened irregularly, the size of the mass varies, and the shape is diverse (20). The boundary is less clear, and enhancement displaying uneven enhancement (21); (III) rhabdomyosarcoma, which is more common in children, has high malignancy, early metastasis, and poor prognosis. It is a grape-like lobulated mass (22,23), and enhancement displays significantly strengthened; (IV) lymphoma is more common in middle-aged and elderly women, with a wide range of lesions (24), uniform density, rare necrotic sacs, and enhancement displays mild to moderate enhancement.
In short, CT and MRI demonstrate the BPG has the characteristics of single shot, clear borders, and rich blood supply. Necrosis, cystic changes, and calcification (curved, annular) are often found in BPG. The ultrasound examination for BPG shows low or medium echo mass with blood flow signals. Combined with clinical manifestations, we can make a more accurate preoperative diagnosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by ethics board of Zhongshan Hospital, Fudan University (No. Y2018-193).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhai H, Ma X, Nie W, et al. Paraganglioma of the Urinary Bladder: A Series of 22 Cases in a Single Center. Clin Genitourin Cancer 2017;15:e765-71. [Crossref] [PubMed]
- Alanee S, Williamson SR, Gupta NS. A rare case of non-functioning bladder paraganglioma treated with robotic assisted partial cystectomy. Urol Case Rep 2019;26:100950. [Crossref] [PubMed]
- Romano IJ, Gentile F, Lippolis A. Postmicturition syndrome: a neglected syndrome dangerous for the bladder and the heart. J Am Soc Hypertens 2018;12:589-93. [Crossref] [PubMed]
- Lazareth H, Cohen D, Vasiliu V, et al. Paraganglioma of the bladder in a kidney transplant recipient: A case report. Mol Clin Oncol 2017;6:553-5. [Crossref] [PubMed]
- Gkikas C, Ram M, Tsafrakidis P. Urinary Bladder Paraganglioma and Concomitant Metastatic Lung Cancer. A Case Report. Urol Case Rep 2016;5:17-9. [Crossref] [PubMed]
- Bosserman AJ, Dai D, Lu Y. Imaging Characteristics of a Bladder Wall Paraganglioma. Clin Nucl Med 2019;44:66-7. [Crossref] [PubMed]
- Liang J, Li H, Gao L, et al. Bladder Paraganglioma: Clinicopathology and Magnetic Resonance Imaging Study of Five Patients. Urol J 2016;13:2605-11. [PubMed]
- Priyadarshi V, Pal DK. Paraganglioma of urinary bladder. Urol Ann 2015;7:402-4. [PubMed]
- Feng N, Li X, Gao HD, et al. Urinary bladder malignant paraganglioma with vertebral metastasis: a case report with literature review. Chin J Cancer 2013;32:624-8. [Crossref] [PubMed]
- Lu H, Male M, Jiang K, et al. Clinical significance of functional and anatomical classifications in paraganglioma of the urinary bladder. Urol Oncol 2019;37:354.e9-17. [Crossref] [PubMed]
- Zhang B, Fu Z, Liu L, et al. Non-functional paraganglioma of urinary bladder managed by transurethral resection. Int Braz J Urol 2019;45:910-5. [Crossref] [PubMed]
- Beilan JA, Lawton A, Hajdenberg J, et al. Pheochromocytoma of the urinary bladder: a systematic review of the contemporary literature. BMC Urol 2013;13:22. [Crossref] [PubMed]
- Deng JH, Li HZ, Zhang YS, et al. Functional paragangliomas of the urinary bladder: a report of 9 cases. Chin J Cancer 2010;29:729-34. [Crossref] [PubMed]
- Quist EE, Javadzadeh BM, Johannesen E, et al. Malignant paraganglioma of the bladder: a case report and review of the literature. Pathol Res Pract 2015;211:183-8. [Crossref] [PubMed]
- Frantellizzi V, Pontico M, Letizia C, et al. Bladder wall paraganglioma located using (123)I-mIBG SPECT and CT imaging. Rev Esp Med Nucl Imagen Mol 2018;37:253-4. [Crossref] [PubMed]
- Asbury WL Jr, Hatcher PA, Gould HR, et al. Bladder pheochromocytoma with ring calcification. Abdom Imaging 1996;21:275-7. [Crossref] [PubMed]
- Li Y, Guo A, Tang J, et al. Evaluation of sonographic features for patients with urinary bladder paraganglioma: a comparison with patients with urothelial carcinoma. Ultrasound Med Biol 2014;40:478-84. [Crossref] [PubMed]
- Chung AD, Schieda N, Flood TA, et al. Suburothelial and extrinsic lesions of the urinary bladder: radiologic and pathologic features with emphasis on MR imaging. Abdom Imaging 2015;40:2573-88. [Crossref] [PubMed]
- Asa SL, Ezzat S, Mete O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J Clin Med 2018. [Crossref] [PubMed]
- Scheunemann D, Pradhan AK, Das SK, et al. Wnt7a and miR-370-3p: new contributors to bladder cancer invasion. Biotarget 2018. [Crossref] [PubMed]
- Spessoto LC, Vasilceac FA, Padilha TL, et al. Incidental Diagnosis of Nonfunctional Bladder Paraganglioma. Urol Case Rep 2015;4:53-4. [Crossref] [PubMed]
- Wong-You-Cheong JJ, Woodward PJ, Manning MA, et al. From the Archives of the AFIP: neoplasms of the urinary bladder: radiologic-pathologic correlation. Radiographics 2006;26:553-80. [Crossref] [PubMed]
- Loveys FW, Pushpanathan C, Jackman S. Urinary Bladder Paraganglioma: AIRP Best Cases in Radiologic-Pathologic Correlation. Radiographics 2015;35:1433-8. [Crossref] [PubMed]
- Mahfoud T, Tanz R, Mesmoudi M, et al. Primary non-Hodgkin's lymphoma of the bladder: case report and literature review. Pan Afr Med J 2013;15:136. [Crossref] [PubMed]


