
Optimization of opioid utility in cancer pain populations
Introduction
Prevalence of opioid use and overdose
Opioid analgesics are generally used as first-line treatment for patients with moderate or severe cancer pain, which is found in nearly 52% of patients with advanced cancer, and in 38% of all cancer patients according to a systematic review (1). These levels of pain are even higher—66%—in patients with metastatic disease (1) and tend to worsen with progression (2). One study in Taiwan observed that more than half (53.2%) of cancer patients were prescribed strong opioids during the 3 months before death (3), while in the United States, 13.3% of cancer survivors 65 years or older reported prolonged use at 5 years post-diagnosis (4).
Between 2001 and 2013, general prescribing of opioids increased all around the globe except in Africa and South Asia (5). Compared with other regions, Asia showed high rates of cancer but only moderate consumption of narcotics (5). In 2009 alone, North American and European countries reportedly consumed 90% of the world’s supply of morphine (6), suggesting that some patients in other regions might not be receiving adequate relief. In response to these global trends in opioid utilization, the World Health Organization (WHO) is currently revising their original three-step pain ladder to include new assessments and delivery methods for cancer patients (7,8).
Overall trends in opioid use may be complicated by the decrease in prescriptions that has been driven by increased regulation and stigmatization in some regions. A retrospective review of 750 patients referred to palliative care at MD Anderson Cancer Center in Texas revealed that the morphine equivalent daily dose (MEDD) decreased by nearly half (from 78 to 40 mg/day; P=0.001) between 2010 and 2015 (9). While there are concerns over opioid misuse and overdose, it should be noted that opioid-associated deaths are 10 times less likely to occur in cancer patients than the non-cancer population. An analysis of the U.S. National Center for Health Statistics data from 2006 to 2016 retrieved 895 and 193,500 opioid-associated deaths in these two populations, respectively (10). Compared with those who died from opioids in the general population, cancer patients who died from opioids were more likely to be female, older and more educated, and less likely to be single (all P<0.001) (10). Accordingly, the 2006 to 2016 opioid-associated death rates increased from 0.52 to 0.66 per 100,000 among cancer patients, and from 5.33 to 8.97 per 100,000 in the general population (10).
A number of common medication problems must be overcome if we are to adequately meet the needs of cancer patients, including inappropriate dosing and opioid combinations, improper dose titration, and failure to prescribe indicated non-opioid co-analgesics (7). The epidemic of opioid overdose in recent years has been well publicized, but prevalence data on overdoses in cancer patients specifically are lacking. However, a propensity-matched analysis of 15,991 U.S. inpatients who underwent elective surgery found much greater odds (382%) of in-hospital opioid overdose among those with cancer pain versus those without it [adjusted odds ratio (OR) 4.82; 95% confidence interval (CI): 2.68–8.67] (11).
Balancing improved pain management with minimal risk of harm
In their discussion of recent guidelines for opioid use, Asthana et al. (6) emphasized that risk-benefit assessment and treatment strategies for cancer patients should be evidence-based, and not simply extrapolated from studies of chronic pain. The recommendation by the 2017 Canadian guideline for chronic non-cancer pain (12) to postpone opioid therapy until psychiatric conditions are stabilized could have unforeseen negative effects in cancer patients in the palliative care setting, who already have a higher prevalence of some mental health disorders (13).
While cancer patients, like non-cancer patients, are at risk of opioid misuse, it is also common for cancer survivors to experience chronic pain long after treatment has ended (14). Long-term opioid use (5 years post-diagnosis) in older cancer survivors has been associated with multiple factors, including female sex, residence in an urban location, lung cancer, and history of depression (4).
It is apparent that some physicians are reluctant to prescribe appropriate doses of opioids, in part due to inadequate knowledge, misconceptions about opioid dependence, and fear of adverse events (6,15). This may be particularly true of physicians working in primary care (6).
As Gaertner et al. (16) pointed out, simple efforts to better educate patients can help to prevent overlapping prescriptions from multiple healthcare teams, minimize use of opioids with other psychotropic agents, and encourage more discreet storage and disposal methods.
Multi-modal personalized management
An overview of guidelines for opioid treatment of chronic non-cancer pain revealed that an individualized management approach is considered helpful to determine the course of treatment (17). Patients with cancer-related pain, which by its nature is constantly evolving, should be comprehensively assessed to identify underlying cause(s) and the presence of any cancer pain syndromes (18). The management of such pain with strong opioids requires the consideration of many factors and the well-informed involvement of patients, their family members, and the entire healthcare team. These factors include the patient’s physiology and genetics, comorbidities and demographics, and also the consideration of a range of bio-psycho-social characteristics of chronic pain that may be poorly understood by many physicians (2,19). Assessment of patients for psychiatric and major social challenges, history of substance abuse or dependence, and poor coping skills may lower the risks of opioid misuse and abuse (19).
There is variability in the proper dosing and effects of opioids between individuals, and long-term treatment can lead to overlapping signs of tolerance, dependence, addiction and abuse (19). Unfortunately, because of the paucity of data on responders, every long-term opioid prescription must be looked upon as an individual trial (19). An updated Cochrane Review of morphine, for example, revealed how few randomized trials of morphine have been conducted, and that most had recruited less than 100 participants (2). Nevertheless, they concluded that morphine taken by mouth produced good pain relief for most people with moderate or severe cancer pain. In Table 1 and the following sections, we outline important considerations and best practices for personalized treatment of patients with cancer pain.
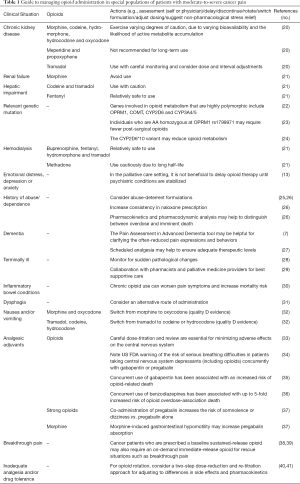
Full table
Demographic considerations
Certain patient subpopulations have been associated with opioid prescribing biases and different treatment outcomes. Higher doses of opioids increase risks of misuse, overdose, hospitalization and suicide (42).
Age, gender, ethnicity and psychology
In a prospective study of a US pain registry involving 1,534 cancer outpatients with chronic pain, 86% of patients received an opioid during at least one clinic visit (43). Patients of a younger age (≤65 years) and male sex were more likely to be prescribed an opioid, with male patients nearly twice as likely as female patients to receive a higher dose (43). In another US study of chronic (non-cancer) pain, both black (vs. white) race and female gender were associated with significantly lower doses of at least 30% less (OR 1.82, 95% CI: 1.22–2.70 and OR 1.43, 95% CI: 1.11–1.83, respectively), consistent with previous studies (42).
Efforts to examine gender differences linked to opioid-related problems have yielded mixed results, and it is unclear how they apply to cancer patients specifically. A review of opioid use in non-cancer pain sufferers revealed that far more women than men use long-term opioids, and make more frequent visits to the clinic (44). Among a population of opioid abusers with chronic pain, women tended to be more depressed and socially and physically impaired by their pain, whereas men reported more aberrant drug-use behaviors and consequences (45). In one study of adults with chronic pain and anxiety sensitivity (fear of symptoms and sensations of anxiety), there were stronger relationships between anxiety sensitivity and opioid misuse or dependence in males compared to females, regardless of income, education, age and severity of pain (46).
Regarding population pharmacokinetics, a study on clearance of oxycodone in 89 patients with cancer pain showed that age, body weight, body surface area, sex and creatinine clearance were all significant covariates, with body surface area being the most important determinant (47).
Respiratory distress induced by opioids can pose a life-threatening event with known risk factors including overdose, older age, sleep apnea, congestive heart failure, chronic obstructive pulmonary disease, and renal failure and dialysis (48). A spontaneous reporting system in Japan yielded data on these risk factors for nine different opioids in more than a million cancer patients (48). Consistent with a previous systematic review and meta-analysis (49), the authors failed to find clinically relevant sex difference in opioid-induced respiratory depression. However, there were higher rates of respiratory events reported for five opioids in patients ≥70 years, underlining the need for careful dosing in older people.
Patient perspective and education
Patients who are stressed and experiencing severe pain may be impaired in decision-making regarding treatment. Interviews with cancer patients and their healthcare providers reveal that signature informed consent may be helpful, and should provide educational materials that match the patient’s literacy level to ensure comprehension. Such consent may not be appropriate in cases of poor prognosis (50).
Metabolic considerations
Liver and hepatic impairments, and hemodialysis
While it is generally understood that cancer patients can develop renal impairment and thus should be assessed before initiating opioid treatment, relatively few studies have reported on renal failure and clinical outcomes in this population. A systematic review of 15 prospective and retrospective, but uncontrolled, studies on the use of opioids in cancer patients with renal impairment concluded that the presence of renal failure should not be a reason to delay the use of an opioid when needed for cancer pain (51). The authors noted that methodological difficulties in studying the use of opioids in cancer include distinguishing between the symptoms of renal failure, comorbidities and opioid use, as well as the presence of individual variability in pharmacological parameters (51). A retrospective analysis of the European Pharmacogenetic Opioid Study of adult patients with advanced cancer who were taking morphine, oxycodone or fentanyl found that mild-to-severely low glomerular filtration rate (GFR) was observed in 40–54% of those on morphine only (52). Patients on morphine who had mild-to-severely low GFR and moderate-to-severely low GFR were more likely to experience severe constipation (P<0.01) and loss of appetite (P=0.04), whereas oxycodone and fentanyl appeared to be safer (52).
In another population with chronic pain (including that from cancer) and chronic kidney disease (CKD), a systematic review of 12 studies concluded that their pain was not being effectively managed, probably due to under-prescribing of analgesics or opioids (20). The authors made several recommendations for specific opioids in CKD patients: (I) morphine, codeine, hydromorphone, hydrocodone and oxycodone should be used with varying degrees of caution, due to varying bioavailability and the likely accumulation of active metabolites (e.g., glucuronides and glucosides). Codeine metabolites include morphine-3-glucuronide and morphine-6-glucuronide; hydromorphone is metabolized to hydromorphone-3-glucuronide and hydromorphone-3-glucoside; oxycodone is metabolized to noroxycodone, oxymorphone and their glucuronides (53); oxymorphone has shown 60% increased bioavailability among patients with renal impairment; (II) meperidine and propoxyphene should not be used long term in patients with or without CKD due to adverse events; (III) methadone pharmacokinetics seem unaffected in this population, but has a long half-life (~36 hours) and thus should be used cautiously because it is poorly removed by hemodialysis; (IV) tramadol has active metabolites that are excreted primarily by the kidney and should be used with careful monitoring, while dose adjustment (e.g., increased between-dose intervals) may be considered; (V) tapentadol may be used in mild-to-moderate renal insufficiency; and (VI) transdermal fentanyl and transdermal buprenorphine seem least affected by CKD, with the former having no active metabolites and the latter having only one-third of its metabolites excreted by the kidneys (20). Amongst those undergoing hemodialysis, it has been suggested that buprenorphine, fentanyl, hydromorphone and tramadol (at doses up to 200 mg/day) may be used (21). In a more recent literature review that analyzed data from special subpopulations of cancer patients, the authors stressed the importance of quality of life and the avoidance of inadequate pain management (21). For patients with hepatic impairment, they recommended fentanyl as a relatively safe option, but that codeine and tramadol may not be, while other opioids should be used with caution.
Genetic polymorphisms
Pharmacogenetic research has revealed that inter-individual variability in analgesic response, adverse effects and addictive vulnerability to opioids is exerted through multiple mechanisms, including polymorphic gene variation and epigenetic regulation. Many genes involved in opioid metabolism are highly polymorphic (54), and include genes that affect the pharmacodynamics, such as µ-opioid receptor (OPRM1) and catechol-o-methyltransferase (COMT), as well as members of the cytochrome P450 superfamily of enzymes (CYP2D6, CYP3A4/5), which are implicated in the pharmacokinetics of opioids (22).
People known as poor metabolizers carry deletions or two null variants that inactivate the function of CYP2D6, which is involved in the metabolism of numerous drugs (55). Despite the low frequency of poor metabolizers among Chinese and Japanese people (~1%) compared with that for Caucasians (7.7%), Asian populations display lower mean CYP2D6 activity than Caucasians (55). This difference has been attributed to the reduced activity of variant CYP2D6*10, which was found in 52.6% of patients in a study of Hong Kong Chinese people (24).
Choi et al. (23) conducted a meta-analysis of 51 studies on post- and intra-operative opioid use and patient genotyping to begin discerning the effects of the vast array of available information on single nucleotide polymorphisms (SNPs). Although the authors cautioned that investigations of SNPs are insufficient to encompass the multiple biochemical pathways that influence opioid requirements, they were able to draw broad distinctions. Individuals who were homozygous for AA at rs1799971, the most widely studied SNP of OPRM1, required less post-surgical opioids than those who were homozygous for GG (P=0.001), which is common (10–20%) in Chinese and Japanese populations. Polymorphisms in CYP2D6, CYP3A4, CYP3A5, COMT, UGT2B7 or ACBC1 did not affect opioid requirements. In the absence of a publicly accessible database of controlled trial results, the authors advocate the application of whole genome sequencing analysis to help guide appropriate opioid selection and dosage (23).
Neuropsychopharmacological considerations
Psychological and cognitive status
While there are many guidelines on use of opioids to address acute and chronic pain, the recommendations may not be appropriate for patients with cancer pain. For example, Asthana et al. (6) highlighted the fact that the 2017 Canadian Guideline on chronic non-cancer pain recommendation to stabilize psychiatric disorders before initiating opioid therapy could inadvertently exclude cancer patients from receiving needed treatment.
The emotional distress that is consistently associated with cancer pain has also been significantly negatively correlated with length of survival, and is known as the sixth vital sign (56). One might argue that the achievement of effective pain management in such patients could improve the psychological state and vice versa. It is worth asking then whether psychological assessment of these common co-morbidities of anxiety and depression in cancer patients are predictive and thus helpful in deciding whether to initiate opioid therapy. In one study of cancer outpatients who had recently taken opioids, scores of self-efficacy (a cognitive dimension of the pain experience) were found to be related to emotional status in terms of both anxiety and depression (56). These results reiterate the observation that in cancer patients who can confidently communicate their pain experience, analgesic treatment can help them gain improvements in emotional health.
It remains unclear whether the use of opioids can have adverse cognitive effects in older adults. A systematic review of 10 studies of adults ≥65 years taking opioids for cancer/chronic non-cancer pain assessed the patients for 14 cognitive domains (57): six studies showed no effect, while four showed mixed (improvements and impairments) effects of opioids on cognitive function, mainly when higher mean doses were used (120–190.7 mg oral MEDD). While acknowledging the methodological challenges of the included studies, the authors concluded that low daily doses of opioids are effective for pain relief in this population (57).
In patients with existing dementia, who may have fewer pain behaviors or not able to verbalize their pain, specific tools like The Pain Assessment in Advanced Dementia (7) may be helpful. The authors of an end-of-life study of older adults with dementia and cancer pain concluded that fear of adverse side effects should not prevent patients with cognitive impairment from being treated for pain (27). Furthermore, they recommended scheduled analgesia to ensure therapeutic levels in patients with known painful conditions who cannot communicate their pain.
Minimizing risks of overdose and abuse
In the U.S. National Cancer Institute Centers, screening questionnaires and urine drug screens suggest that ≥1 in 5 patients with cancer may be at risk of opioid-use disorder (58). Chemical coping, in which people use opioids to deal with stressful life events, can put patients at risk of complications including neurotoxicities and respiratory depression (59). In a US prospective study of 432 patients with advanced cancer, 18% of the patients were identified by palliative medicine specialists as chemically coping, but only 4% were officially documented as such (59). In an Australian chart review of 398 adolescent and young adults, most of whom were oncology patients, 94 received opioid therapy, of which 11.7% exhibited aberrant opioid-associated behaviors that suggested misuse. Importantly, most of these patients (90.9%) had at least one psychosocial risk factor (60). These results create an imperative to improve assessment and reporting to prevent the common misuse of opioids in this population.
High dose-variability has also been identified as a possible risk factor for overdose. In a nested case control study involving nearly 15,000 patients prescribed long-term opioids, researchers analyzed the effect of dose variability up to and exceeding a standard deviation >27.2 mg of morphine equivalents (61). They found >3-fold greater risk of overdose in patients who experienced high variability in dosing, even among those receiving low doses. Thus, these findings suggest that caution be used when attempting to modify opioid dosing. Furthermore, non-compliance with prescribed regimens among cancer patients was significantly associated with a history of alcohol use, anxiety, a high score on a short-form self-reporting questionnaire on aberrant medication behaviors (SOAPP-SF), and younger age (46±12 years) in a US cancer clinic study (62).
Harm-reduction strategies for reducing the risk of opioid abuse in cancer patients include an array of abuse-deterrent drug formulations (ADFs) as well as increased consistency in prescribing the opioid-antagonist naloxone (26). Some ADFs limit physical and chemical manipulation of pills and tablets, while others involve aversion technologies or delivery systems (e.g., subcutaneous implants) that make it harder to abuse. However, ADFs mean higher costs for patients, yet they are no less addictive, and cannot guarantee absolute avoidance of abuse (26). As a strategy to reverse intentional or unintentional opioid overdose, naloxone is widely recommended as relatively safe and effective by the WHO and other guidelines (26). However, it should be noted that signs and symptoms of opioid intoxication may be difficult to confirm in patients who are terminally ill from end-stage cancer. Pharmacokinetics and pharmacodynamic analysis of the opioid in question may help to distinguish between overdose and imminent death (26).
Risks of co-administration of opioids and analgesic adjuvant
A review of pharmacological management of cancer pain concluded that the use of analgesic adjuvants: gabapentin, pregabalin, amitriptyline or duloxetine should be considered for cancer pain patients having a neuropathic component and who are only partially responsive to opioids (33). However, the combination of an opioid with any of these drugs may lead to central nervous system symptoms, thus careful dose titration and review are essential (33). Co-administration of pregabalin with a strong opioid increases the risk of somnolence or dizziness compared with pregabalin alone, whereas co-administration of morphine and gabapentin may result in increased gabapentin absorption due to morphine-induced gastrointestinal hypomotility (37). In a nested case control study of non-cancer patients in Canada, the concurrent use of gabapentin also increased the risk of opioid-related deaths—by 49% (35). Additionally, the US Food and Drug Administration (FDA) recently warned that serious breathing difficulties may occur in patients on central nervous system depressants, including opioids, who are also taking gabapentin or pregabalin (34).
The concurrent use of opioids with benzodiazepines, which is still common in cancer patients, has been associated with a risk of overdose-associated death up to 5-fold greater than for opioids alone (36).
Cancer type, stage, performance and pain level
Cancer type, stage and prior therapy
A cohort of >100,000 military veteran survivors of common cancer types was used to examine multiple factors associated with persistent opioid use in cancer patients (63). Stratified analyses revealed that patients with more advanced stages of colon, lung, or head and neck cancer had increased odds of persistent opioid use compared to those at earlier stages. Prostate and lung cancer patients who received radiation therapy had increased opioid use compared with definitive surgery, while renal cancer patients receiving chemotherapy had increased use compared to those with no chemotherapy (63). In Taiwan, where opioids are consumed at lower levels compared with global figures, retrospective data from 162,679 cancer outpatients [44.9% with gastrointestinal (GI) cancer] were analyzed for patterns of strong opioid use in the final 12 months of life (3). Prevalence of strong opioid prescription and severity of pain were associated with disease stage, and were highest in patients with progressive disease. Patients with head and neck cancer were most likely to be prescribed strong opioids, while those with GI cancer were least likely (3). A recent systematic review of 74 studies on cancer-related pain found that lung, head and neck, breast and prostate cancers were associated more with somatic pain, whereas colorectal, gastric, liver, pancreatic and uterine cancers were associated more with visceral pain (64). Patients with lung or gastrointestinal cancer responded worse to opioids compared to other cancers (64).
In a retrospective study of newly diagnosed stage IV cancer patients, opioid use (≥5 mg oral MEDD) was associated with greater healthcare utilization but shortened survival, adjusted for age, gender and prognostic group (65). One possibility for these results is that opioid activation of peripheral mu opioid receptors (MOP-R) actually stimulated tumor growth. The authors proposed future prospective studies to explore ways to mitigate this potentially negative impact of opioids while maintaining adequate pain control (65).
Occasionally in patients at the terminal stage of cancer, debilitation from disease progression may increase the risk of respiratory depression onset, which may require opioid antagonist, dose reduction or switching (28). While proper use of opioids was safe for nearly all 2,443 terminal cancer patients in the study, the results highlight the importance of pharmacotherapy monitoring of sudden pathological changes in this population (28).
Comorbidities and disease progression
In patients with GI disease, chronic opioid use can worsen not only the disease but also pain symptoms, as well as increase the mortality risk of those with inflammatory bowel disease (30). The mechanism for this, and the development of opioid tolerance, may be related to changes in the microbiome (30).
The correlation between cholesterol level and opioid analgesia have been previously reported, attributed to the location of OPRM1 in cholesterol-rich areas of the cell membrane (66). Interestingly, a retrospective study of lung cancer patients reported that those with low cholesterol levels were less likely to respond to an initial dose of three different types of opioids than those with high cholesterol (66).
For people with multiple serious co-morbidities, such as hepatic and renal dysfunction, avoiding adverse events while ensuring adequate pain management is challenging. Collaboration between pharmacists and palliative medicine providers may offer end-of-life patients the best possible care (29).
Pain types and levels
Cancer pain is heterogeneous, which may arise from pathological changes including bone and visceral metastases, as well as diagnostic and therapeutic interventions such as radiotherapy and/or chemotherapy (40). Concept such as nociceptive (somatic and/or visceral), neuropathic (peripheral and/or central), mixed and breakthrough pain are important in the assessment of cancer patients (67). While cancer pain driven by neuropathic mechanisms may be a negative predictor of pain therapy (68) and has generally been considered to be resistant to opioids or require adjuvant analgesics for controlling mixed pain (18), this notion has recently been challenged by results in patients with mixed nociceptive-neuropathic cancer pain (69). In a prospective study of 240 cancer patients, 72.1% of patients had nociceptive pain alone and 27.9% had mixed pain. Both patients with mixed pain and purely nociceptive pain responded to opioids with significant reductions in pain intensity (69).
In a US prospective study of 1,885 patients at 12 oncology units, the incidence of chronic cancer pain at 6-month after the first visit ranged from 13–28% depending on primary tumor location, of which 19.9% had neuropathic pain (70). Chronic pain was present in >30% of patients with breast, colorectal, head/neck, lung, gynecological or prostate cancer; and neuropathic pain was present in 7.4%, 6.9% and 6.9% of patients with breast, head/neck and lung cancer, respectively (70). Patients with chronic cancer pain with neuropathic characteristics were more likely to be treated with antidepressants and/or antiepileptic drugs than those without (52.9% vs. 6.9%), including in combination with opioids (24.6% overall).
National Comprehensive Cancer Network Guidelines stipulate that all patients with cancer should be screened for pain throughout the treatment period. Pain intensity must be quantified and re-assessed at specified intervals, and qualitative characteristics be elicited if possible, using patient-reported numerical, categorical or pictorial scales, as appropriate (71). It is also crucial to provide psychosocial support and offer integrative interventions at every pain level (71).
Considerations for titration, rotation and conversion
Immediate-release (IR) vs. sustained-release (SR) formulations
In terms of dose-titration and the benefit of more stable pharmacokinetic profiles (72), SR oxycodone was associated with more efficient and better tolerated dose-titration compared with IR morphine in a multicenter randomized controlled (73). Conversely, as argued by the European Association for Palliative Care (EAPC), IR formulations may be helpful for rapidly achieving adequate analgesia (74). Cancer patients who are prescribed a baseline SR opioid may also require an on-demand IR opioid for rescue situations such as breakthrough pain, and these need not necessarily be the same medication (38,39).
The selection of an appropriate on-demand IR opioid depends on a number of factors: the patient’s physical condition and dexterity (which affect administration and tolerance) and likelihood of adherence/abuse; and the medication’s onset and duration of action and route of administration (38,75). For example, oral tramadol may be efficacious in cancer patients experiencing breakthrough pain of mild to moderate intensity (76); fentanyl may provide a greater level of pain relief in a shorter time frame (77). Because of a lack of bioequivalence between different fentanyl formulations, individualized dose-titration may be needed for balancing efficacy with tolerability and avoiding overdose (75). The possibility of an end-of-dose failure should also be considered, in which case opioid rotation may be a better option (38). In a prospective, cross-sectional study of breakthrough cancer pain, Magnani et al. (78) found that the factors that most influenced prescription with oral or intravenous morphine, or transmucosal IR fentanyl, were the baseline opioid dosage, home-care setting of assistance, and self-ability to take medication.
A Cochrane review of oral morphine efficacies found no difference in pain control between IR and modified-release preparations (which are currently the standard for cancer pain relief) (2). Another Cochrane review showed no significant differences in adverse events between oxycodone IR and controlled-release and that three of four studies showed similar treatment results; however, the risks of bias and number of events/participants in the included studies lowered the reliability of the evidence (79).
It should be noted that some extended-release formulations are designed for deterring abuse and preventing unintentional misuse, e.g., by patients who crush their pills to aid swallowing (25). For patients with known dysphagia, which was found in 5–20% of chronic pain patients in a US survey of patients and physicians (31), the route of administration must be considered. Transdermal SR fentanyl is one option that may be helpful when the oral route is not available (80,81).
A review aimed at guiding the use of oxycodone suggested that the prolonged release formulation may present less potential for abuse than the IR formulation (82). However, patients on SR formulations of opioids may also be prone to take them more frequently than recommended, in part due to end-of-dose failure (83).
Switching considerations
Because there is a wide range of responses to opioids—e.g., analgesia, tolerance, dosage needs and adverse effects all vary—efforts are needed to evaluate the efficacy of a specific drug in the individual patient. Corli et al. (84) devised a methodology to apply two parameters (changes in pain intensity and opioid daily dose) to categorize good responders from bad responders among cancer patients with moderate-to-severe pain. In their Italian study of 201 cancer patients on strong opioids, 63.7% had a positive analgesic response and 80.1% had a dose-related positive response, while only 55.2% of cases were found to be positive for both (84).
Opioid rotation may be indicated by inadequate analgesic response, adverse effects, tolerance, opioid induced hyperalgesia, or change in clinical state (e.g., kidney failure) that impair the pharmacokinetics of the medication (41). In a phase 4 study of 498 patients receiving strong opioids for cancer pain, the main reasons for opioid switching were: uncontrolled pain (52.3%), severe adverse effects (22.1%), both pain and adverse effects (4.7%), and dysphagia (20.9%) (85); there was significantly more neuropathic or mixed pain in the switching vs. non-switching population. Half (51.45%) of the patients who underwent switching experienced improved relief of pain and 43.5% had reduced side effects (85).
Mercadante et al. (68,86) developed an assessment tool for evaluating neuropathic pain. The rates of opioid switching or rotation, where one opioid is substituted for another in order to improve pain relief or reduce side effects, have been reported to range from 21–44% in cancer pain (86). Although this is a high rate and may be attributed to the prevalence of difficult cases in cancer pain, it is effective in 50–90% of patients (86).
For the management of opioid-induced nausea and vomiting, which occurs in 40% and 15–25% of chronic pain patients, a systematic review by the EAPC uncovered a paucity of high-quality data (32). The authors reiterated previous calls for better studies, but provided two (albeit weak) recommendations for switching in cancer patients with nausea: switch from (I) morphine to oxycodone (quality D); and (II) tramadol to either codeine or hydrocodone (quality D) (32).
Regarding equianalgesic (equivalent dose) MEDD for opioids, there is currently no consensus recommendation (6), with variation in conversion factors across multiple guidelines (87). In the meantime, the two-step rotation proposed by Fine and Portenoy (40) is a feasible approach: (I) dose decrease of 25–50% of the new opioid with some exceptions (75–90% for methadone); and (II) an additional adjustment of 15–30% according to the individual patient demographics and condition. Interestingly, various guidelines also suggest similar two-step dose-reduction and re-titration approaches. For example, the EAPC 2012 guideline recommends that for rotation due to inadequate analgesia and/or severe side effects, the dose of the second-line opioid should be lower than the calculated dose, followed by re-titration depending on clinical effect (88,89). The National Health Services Scotland Palliative Care Guideline (89,90) recommends dose-reduction of up to 30% followed by re-titration, for adjusting to differences in pharmacokinetics, pharmacodynamics and tolerance, and/or in patients who are opioid-toxic, frail or elderly.
Conclusions
Optimizing safe, consistent pain management with opioids while minimizing adverse effects and potential for misuse is achievable for cancer patients, even in the current climate. A multi-modal, personalized approach that incorporates the bio-psycho-social concept of pain is instrumental toward achieving this goal. The type and dose of opioid, as well as the treatment course, should be selected for individual patients based on probable efficacy, safety, and minimal side effects. As cancer rates rise in parallel with advanced treatments that allow patients to live longer, updated pain management guidelines that draw from well-designed clinical trials are urgently needed to ensure that patients with cancer pain are managed appropriately.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mellar P. Davis) for the series “Opioid Utility the Other Half of Equianalgesia” published in Annals of Palliative Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Opioid Utility the Other Half of Equianalgesia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 2016;51:1070-90.e9. [Crossref] [PubMed]
- Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst Rev 2016;4:CD003868. [PubMed]
- Lin YL, Hsieh RK, Tang CH. Strong opioid prescription in cancer patients in their final year of life: A population-based analysis using a Taiwanese health insurance database. Asia Pac J Clin Oncol 2018;14:e498-504. [Crossref] [PubMed]
- Shah R, Chou LN, Kuo YF, et al. Long-Term Opioid Therapy in Older Cancer Survivors: A Retrospective Cohort Study. J Am Geriatr Soc 2019;67:945-52. [Crossref] [PubMed]
- International Narcotics Control Board. Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes. New York: United Nations, 2016.
- Asthana R, Goodall S, Lau J, et al. Framing of the opioid problem in cancer pain management in Canada. Curr Oncol 2019;26:e410-3. [Crossref] [PubMed]
- WHO. Guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Geneva: World Health Organization, 2018.
- WHO revision of pain management guidelines. Geneva: World Health Organization, 2019.
- Haider A, Zhukovsky DS, Meng YC, et al. Opioid Prescription Trends Among Patients With Cancer Referred to Outpatient Palliative Care Over a 6-Year Period. J Oncol Pract 2017;13:e972-81. [Crossref] [PubMed]
- Chino FL, Kamal A, Chino JP. Opioid-associated deaths in patients with cancer: A population study of the opioid epidemic over the past 10 years. J Clin Oncol 2018;36:230. [Crossref]
- Onyeakusi NE, Mukhtar F, Gbadamosi SO, et al. Cancer-Related Pain Is an Independent Predictor of In-Hospital Opioid Overdose: A Propensity-Matched Analysis. Pain Med 2019;20:2552-61. [Crossref] [PubMed]
- Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189:E659-66. [Crossref] [PubMed]
- Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. The Lancet Oncology 2011;12:160-74. [Crossref] [PubMed]
- Jiang C, Wang H, Wang Q, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain in Cancer Survivors in the United States. JAMA Oncology 2019;5:1224-6. [Crossref] [PubMed]
- Pautex S, Vogt-Ferrier N, Zulian GB. Breakthrough pain in elderly patients with cancer: treatment options. Drugs Aging 2014;31:405-11. [Crossref] [PubMed]
- Gaertner J, Boehlke C, Simone CB II, et al. Early palliative care and the opioid crisis: ten pragmatic steps towards a more rational use of opioids. Ann Palliat Med 2019;8:490-7. [Crossref] [PubMed]
- Cheung CW, Qiu Q, Choi SW, et al. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician 2014;17:401-14. [PubMed]
- Yoong J, Poon P. Principles of cancer pain management: An overview and focus on pharmacological and interventional strategies. Aust J Gen Pract 2018;47:758-62. [Crossref] [PubMed]
- Stein C, Kopf A. Pain therapy - Are there new options on the horizon? Best Pract Res Clin Rheumatol 2019;33:101420. [Crossref] [PubMed]
- Nagar VR, Birthi P, Salles S, et al. Opioid Use in Chronic Pain Patients with Chronic Kidney Disease: A Systematic Review. Pain Med 2017;18:1416-49. [Crossref] [PubMed]
- Vieira C, Bras M, Fragoso M. Acta Med Port 2019;32:388-99. [Opioids for Cancer Pain and its Use under Particular Conditions: A Narrative Review]. [Crossref] [PubMed]
- V Subramaniam A. Salem Yehya AH, Oon CE. Molecular Basis of Cancer Pain Management: An Updated Review. Medicina (Kaunas) 2019. [Crossref]
- Choi SW, Lam DMH, Wong SSC, et al. Effects of Single Nucleotide Polymorphisms on Surgical and Postsurgical Opioid Requirements: A Systematic Review and Meta-Analysis. Clin J Pain 2017;33:1117-30. [PubMed]
- Garcia-Barceló M, Chow LY, Chiu HF, et al. Genetic analysis of the CYP2D6 locus in a Hong Kong Chinese population. Clin Chem 2000;46:18-23. [Crossref] [PubMed]
- Gudin J, Levy-Cooperman N, Kopecky EA, et al. Comparing the Effect of Tampering on the Oral Pharmacokinetic Profiles of Two Extended-Release Oxycodone Formulations with Abuse-Deterrent Properties. Pain Med 2015;16:2142-51. [Crossref] [PubMed]
- Mitchell MT. Dancing with Deterrents: Understanding the Role of Abuse-Deterrent Opioid Formulations and Naloxone in Managing Cancer Pain. Oncologist 2019;24:1505-9. [Crossref] [PubMed]
- Monroe T, Carter M, Feldt K, et al. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J Adv Nurs 2012;68:2070-8. [Crossref] [PubMed]
- Uekuzu Y, Higashiguchi T, Futamura A, et al. A Clinical Study on Administration of Opioid Antagonists in Terminal Cancer Patients: 7 Patients Receiving Opioid Antagonists Following Opioids among 2443 Terminal Cancer Patients Receiving Opioids. Biol Pharm Bull 2017;40:278-83. [Crossref] [PubMed]
- Brecher DB, West TL. Pain management in a patient with renal and hepatic dysfunction. J Palliat Med 2014;17:249-52. [Crossref] [PubMed]
- Akbarali HI, Dewey WL. The gut-brain interaction in opioid tolerance. Curr Opin Pharmacol 2017;37:126-30. [Crossref] [PubMed]
- Pergolizzi JV, Taylor R, Nalamachu S, et al. Challenges of treating patients with chronic pain with dysphagia (CPD): physician and patient perspectives. Curr Med Res Opin 2014;30:191-202. [Crossref] [PubMed]
- Sande TA, Laird BJA, Fallon MT. The Management of Opioid-Induced Nausea and Vomiting in Patients with Cancer: A Systematic Review. J Palliat Med 2019;22:90-7. [Crossref] [PubMed]
- Wood H, Dickman A, Star A, et al. Updates in palliative care - overview and recent advancements in the pharmacological management of cancer pain. Clin Med (Lond) 2018;18:17-22. [Crossref] [PubMed]
- U.S. Food and Drug Administration. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) when used with CNS depressants or in patients with lung problems. In: Drug Safety Communications. 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med 2017;14:e1002396. [Crossref] [PubMed]
- Haider A, Azhar A, Nguyen K, et al. Concurrent use of opioids with benzodiazepines or nonbenzodiazepine sedatives among patients with cancer referred to an outpatient palliative care clinic. Cancer 2019;125:4525-31. [Crossref] [PubMed]
- Kato H, Miyazaki M, Takeuchi M, et al. A retrospective study to identify risk factors for somnolence and dizziness in patients treated with pregabalin. J Pharm Health Care Sci 2015;1:22. [Crossref] [PubMed]
- Schneider G, Voltz R, Gaertner J. Cancer Pain Management and Bone Metastases: An Update for the Clinician. Breast Care (Basel) 2012;7:113-20. [Crossref] [PubMed]
- Schug SA, Chandrasena C. Pain management of the cancer patient. Expert Opin Pharmacother 2015;16:5-15. [Crossref] [PubMed]
- Fine PG, Portenoy RK. Ad Hoc Expert Panel on Evidence R, et al. Establishing "best practices" for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009;38:418-25. [Crossref] [PubMed]
- Treillet E, Laurent S, Hadjiat Y. Practical management of opioid rotation and equianalgesia. J Pain Res 2018;11:2587-601. [Crossref] [PubMed]
- Buonora M, Perez HR, Heo M, et al. Race and Gender Are Associated with Opioid Dose Reduction Among Patients on Chronic Opioid Therapy. Pain Med 2019;20:1519-27. [Crossref] [PubMed]
- Moryl N, Dave V, Glare P, et al. Patient-Reported Outcomes and Opioid Use by Outpatient Cancer Patients. J Pain 2018;19:278-90. [Crossref] [PubMed]
- Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med 2012;13:1181-211. [Crossref] [PubMed]
- Manubay J, Davidson J, Vosburg S, et al. Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J Addict Med 2015;9:46-52. [Crossref] [PubMed]
- Rogers AH, Manning K, Garey L, et al. Sex differences in the relationship between anxiety sensitivity and opioid misuse among adults with chronic pain. Addict Behav 2020;102:106156. [Crossref] [PubMed]
- Kokubun H, Yoshimoto T, Hojo M, et al. Pharmacokinetics of Oxycodone After Intravenous and Subcutaneous Administration in Japanese Patients with Cancer Pain. J Pain Palliat Care Pharmacother 2014;28:338-50. [Crossref] [PubMed]
- Sugawara H, Uchida M, Suzuki S, et al. Analyses of Respiratory Depression Associated with Opioids in Cancer Patients Based on the Japanese Adverse Drug Event Report Database. Biol Pharm Bull 2019;42:1185-91. [Crossref] [PubMed]
- Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open 2018;8:e024086. [Crossref] [PubMed]
- Giannitrapani KF, Fereydooni S, Azarfar A, et al. Signature Informed Consent for Long-Term Opioid Therapy in Patients With Cancer: Perspectives of Patients and Providers. J Pain Symptom Manage 2020;59:49-57. [Crossref] [PubMed]
- King S, Forbes K, Hanks GW, et al. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med 2011;25:525-52. [Crossref] [PubMed]
- Kurita GP, Lundstrom S, Sjogren P, et al. Renal function and symptoms/adverse effects in opioid-treated patients with cancer. Acta Anaesthesiol Scand 2015;59:1049-59. [Crossref] [PubMed]
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the Management of Chronic Severe Pain in the Elderly: Consensus Statement of an International Expert Panel with Focus on the Six Clinically Most Often Used World Health Organization step III Opioids (Buprenorphine, Fentanyl, Hydromorphone, Methadone, Morphine, Oxycodone). Pain Pract 2008;8:287-313. [Crossref] [PubMed]
- Owusu Obeng A, Hamadeh I, Smith M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017;37:1105-21. [Crossref] [PubMed]
- Shen H, He MM, Liu H, et al. Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos 2007;35:1292-300. [Crossref] [PubMed]
- Ding SA, Liang SY, Wu WW, et al. Opioid-taking self-efficacy as influencing emotional status in patients with cancer pain. Eur J Oncol Nurs 2016;25:77-82. [Crossref] [PubMed]
- Pask S, Dell'Olio M, Murtagh FEM, et al. The effects of opioids on cognition in older adults with cancer and chronic non-cancer pain: A systematic review. J Pain Symptom Manage 2019. [Epub ahead of print]. [PubMed]
- Carmichael A-N, Morgan L, Del Fabbro E. Identifying and assessing the risk of opioid abuse in patients with cancer: an integrative review. Subst Abuse Rehabil 2016;7:71-9. [PubMed]
- Kwon JH, Tanco K, Park JC, et al. Frequency, Predictors, and Medical Record Documentation of Chemical Coping Among Advanced Cancer Patients. Oncologist 2015;20:692-7. [Crossref] [PubMed]
- Ehrentraut JH, Kern KD, Long SA, et al. Opioid misuse behaviors in adolescents and young adults in a hematology/oncology setting. J Pediatr Psychol 2014;39:1149-60. [Crossref] [PubMed]
- Glanz JM, Binswanger IA, Shetterly SM, et al. Association Between Opioid Dose Variability and Opioid Overdose Among Adults Prescribed Long-term Opioid Therapy. JAMA Netw Open 2019;2:e192613. [Crossref] [PubMed]
- Koyyalagunta D, Bruera E, Engle MP, et al. Compliance with Opioid Therapy: Distinguishing Clinical Characteristics and Demographics Among Patients with Cancer Pain. Pain Med 2018;19:1469-77. [Crossref] [PubMed]
- Vitzthum LK, Riviere P, Sheridan P, et al. Predicting Persistent Opioid Use, Abuse and Toxicity Among Cancer Survivors. J Natl Cancer Inst 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Lucenteforte E, Vagnoli L, Pugi A, et al. A systematic review of the risk factors for clinical response to opioids for all-age patients with cancer-related pain and presentation of the paediatric STOP pain study. BMC Cancer 2018;18:568. [Crossref] [PubMed]
- Zylla D, Steele G, Shapiro A, et al. Impact of opioid use on health care utilization and survival in patients with newly diagnosed stage IV malignancies. Support Care Cancer 2018;26:2259-66. [Crossref] [PubMed]
- Huang Z, Liang L, Li L, et al. Opioid doses required for pain management in lung cancer patients with different cholesterol levels: negative correlation between opioid doses and cholesterol levels. Lipids Health Dis 2016;15:47. [Crossref] [PubMed]
- Caraceni A, Shkodra M. Cancer Pain Assessment and Classification. Cancers 2019;11:510. [Crossref] [PubMed]
- Mercadante S, Gebbia V, David F, et al. Tools for identifying cancer pain of predominantly neuropathic origin and opioid responsiveness in cancer patients. J Pain 2009;10:594-600. [Crossref] [PubMed]
- Bechakra M, Moerdijk F, van Rosmalen J, et al. Opioid responsiveness of nociceptive versus mixed pain in clinical cancer patients. Eur J Cancer 2018;105:79-87. [Crossref] [PubMed]
- Bouhassira D, Luporsi E, Krakowski I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 2017;158:1118-25. [Crossref] [PubMed]
- Swarm RA, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2013;11:992-1022. [Crossref] [PubMed]
- Gardner-Nix J, Mercadante S. The role of OROS hydromorphone in the management of cancer pain. Pain Pract 2010;10:72-7. [Crossref] [PubMed]
- Pan H, Shen P, Shu Q, et al. Efficacy and safety of sustained-release oxycodone compared with immediate-release morphine for pain titration in cancer patients: A multicenter, open-label, randomized controlled trial (SOCIAL). Medicine (Baltimore) 2019;98:e15505. [Crossref] [PubMed]
- Klepstad P, Kaasa S, Borchgrevink PC. Starting Step III opioids for moderate to severe pain in cancer patients: Dose titration: A systematic review. Palliat Med 2011;25:424-30. [Crossref] [PubMed]
- Smith HS. Considerations in selecting rapid-onset opioids for the management of breakthrough pain. J Pain Res 2013;6:189-200. [Crossref] [PubMed]
- Husic S, Imamovic S, Matic S, et al. Characteristics and Treatment of Breakthrought Pain (BTcP) in Palliative Care. Med Arch 2017;71:246-50. [Crossref] [PubMed]
- Zeppetella G, Davies A, Eijgelshoven I, et al. A Network Meta-Analysis of the Efficacy of Opioid Analgesics for the Management of Breakthrough Cancer Pain Episodes. J Pain Symptom Manage 2014;47:772-85.e5. [Crossref] [PubMed]
- Magnani C, Giannarelli D, Calvieri A, et al. Breakthrough cancer pain tailored treatment: which factors influence the medication choice? An observational, prospective and cross-sectional study in patients with terminal cancer. Postgrad Med J 2018;94:566-70. [Crossref] [PubMed]
- Schmidt-Hansen M, Bennett MI, Arnold S, et al. Oxycodone for cancer-related pain. Cochrane Database Syst Rev 2017;8:CD003870. [PubMed]
- Mercadante S. Options for Treating Pain in Cancer Patients with Dysphagia. Drugs 2017;77:629-35. [Crossref] [PubMed]
- Othman AH, Mohamad MF, Sayed HA. Transdermal Fentanyl for Cancer Pain Management in Opioid-Naive Pediatric Cancer Patients. Pain Med 2016;17:1329-36. [Crossref] [PubMed]
- Deeks ED, Lyseng-Williamson KA. Oxycodone prolonged release: a guide to its use in the EU. Drugs Ther Perspect 2016;32:363-8. [Crossref]
- Kim DY, Song HS, Ahn JS, et al. The dosing frequency of sustained-release opioids and the prevalence of end-of-dose failure in cancer pain control: a Korean multicenter study. Support Care Cancer 2010;19:297-301. [Crossref] [PubMed]
- Corli O, Roberto A, Greco MT, et al. Assessing the response to opioids in cancer patients: a methodological proposal and the results. Support Care Cancer 2015;23:1867-73. [Crossref] [PubMed]
- Corli O, Roberto A, Corsi N, et al. Opioid switching and variability in response in pain cancer patients. Support Care Cancer 2019;27:2321-7. [Crossref] [PubMed]
- Mercadante S, Bruera E. Opioid switching in cancer pain: From the beginning to nowadays. Crit Rev Oncol Hematol 2016;99:241-8. [Crossref] [PubMed]
- Cheung CW, Chan TC, Chen PP, et al. Opioid therapy for chronic non-cancer pain: guidelines for Hong Kong. Hong Kong Med J 2016;22:496-505. [Crossref] [PubMed]
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58-68. [Crossref] [PubMed]
- Schuster M, Bayer O, Heid F, et al. Opioid Rotation in Cancer Pain Treatment. Dtsch Arztebl Int 2018;115:135-42. [PubMed]
- Scotland N. Scottish Palliative Care Guidelines - Choosing and Changing Opioids. 2019. Available online: https://www.palliativecareguidelines.scot.nhs.uk/guidelines/pain/choosing-and-changing-opioids.aspx


