
MiR-21 antagomir improves insulin resistance and lipid metabolism disorder in streptozotocin-induced type 2 diabetes mellitus rats
Introduction
Diabetes mellitus has become an important public health concern throughout the world because of the sedentary lifestyle and the increasing aging population. Diabetes mellitus is the ninth major cause of death (1), and over 90% of diabetes mellitus cases are type 2 diabetes mellitus (T2DM) (2). What is more, the incidence of T2DM is increasing at a rapid rate (3). T2DM is characterized by insulin resistance, abnormal insulin secretion, or both (4). T2DM may increase the risk of various diseases, including cardiovascular disease and cerebrovascular disease (5,6). Weight control, diet, and oral medications are regular treatments for T2DM (7). However, the existing treatments are lacking long-term effective blood glucose control; a more effective therapeutic strategy needs to be found.
MicroRNAs (miRNAs) are non-coding RNAs that can regulate gene expression. MiR-21 is located on chromosome 17q23-2, and it is an important member of miRNAs (8). Mir-21 is overexpressed in almost all human tumors and plays an important role in the proliferation (9), apoptosis (10), and invasion (11). Besides, miRNAs are involved in various pathogenesis of diabetes and related microvascular and macrovascular complications (12). Meanwhile, inhibition of miR-21 has been reported to be an effective therapy for diabetic nephropathy (13). Nevertheless, the molecular mechanism of miR-21 in treating diabetes is unclear.
Insulin resistance is a major pathogenic factor of T2DM. Insulin maintains glucose homeostasis as an effective regulator of glucose metabolism (14). Insulin resistance means the reduced sensitivity of tissues to insulin-mediated biologic activity (15), which will result in increased adverse events such as obesity, hypertension, and dyslipidemia in patients with T2DM (16). Insulin resistance is related to lipid metabolism disorders in patients with T2DM (17). One of the main mechanisms of insulin resistance may be that lipid metabolism defect leads to damage of the insulin signaling pathway (18). So it is important to improve insulin resistance in the treatment of T2DM.
In the present study, we explored the effect of miR-21 antagomir on streptozotocin (STZ)-induced T2DM rats. We proved that miR-21 antagomir improved insulin resistance and lipid metabolism disorder in STZ-induced D2TM rats by the up-regulation of tissue inhibitor of metalloproteinases 3 (TIMP3). This study may supply experimental data for the mechanism of miR-21 antagomir in the treatment of T2DM.
Methods
Reagents
STZ was bought from Sigma-Aldrich, USA. Insulin Injection was obtained from Nanjing Xinbai Pharmaceutical Co., Ltd.
Animals
Male Sprague-Dawley (SD) rats about 230–250 g were bought from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). The animals were supported in a room with a standardized condition (23±1 °C, 45–55% humidity, 12 h light/dark cycle). Rats were acclimatized for 7 days with free access to water and standard rat nutrients before the experiments. All experiments and protocols were performed according to the guidelines of Animal Use and Care of the National Institutes of Health (NIH).
Induction of T2DM rat model
One hundred and eighty SD rats were randomly divided into two groups: one group of 40 rats was fed a regular diet (healthy group). The other group of 140 rats was fed with a high-fat diet (HFD), HFD consisted of 41% fat, 41% carbohydrate, and 18% protein (T2DM group). After 4 weeks, the T2DM group was intravenously injected with STZ (40 mg/kg) once. STZ was dissolved in 0.01 M sodium citrate (pH adjusted to 4.5). Then blood was collected from the tail vein, and blood glucose was measured in all groups.
Experimental design
The experiment was divided into two phases. In the first phase, 30 T2DM rats were randomly divided into 3 groups (n=10): T2DM group, T2DM rats with miR-21 antagomir group, T2DM rats with NC antagomir group. Ten healthy rats were in the control group, also called the healthy group. Factors related to lipid metabolic disorder and insulin resistance were detected. Then, the target relationship between miR-21 and TIMP3 was measured. After that experiment in phase two began. 30 T2DM rats were randomly divided into 3 groups (n=10): T2DM group, T2DM rats with miR-21 antagomir group, T2DM rats with the si-TIMP3 group. The healthy group still consisted of 10 healthy rats. Every group was perpetuated for 8 weeks. MiR-21 antagomir, NC antagomir, and si-TIMP3 were intravenously injected into rats once every 3 days. All the rats were free to access to water and a regular diet.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with an RNA extraction kit according to the manufacturer’s instructions (Qiagen, Venlo, The Netherlands). Total cDNA was synthesized after equal amounts of RNA were reversely transcribed by a one-step PrimeScript miRNA cDNA synthesis kit (Takara, Dalian, China). The qRT-PCR was performed as a previous report (19). The primer of miR-21was synthesized by Sangon Biotech (Shanghai, China). And qPCR was performed using the ABI Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The expression was calculated using 2−ΔΔCt and normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Biochemical analysis
At the end of experiments, the blood samples were collected in fasting state and frozen at −80 °C for the standby use for measurements of blood glucose, triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-Cho), insulin, adiponectin. The blood glucose was detected by a glucometer (Aquo-Check, Roche). The plasma insulin level was determined by a human insulin ELISA kit (Multi Sciences, China). An enzymatic colorimetric test measured TG, TC, and HbA1c. LDL-Cho and high-density lipoprotein cholesterol (HDL-Cho) levels were detected by a Beckman automatic biochemical analyzer (Roche, Germany). homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the following formula: HOMA-IR = [fasting insulin (U/mL) × fasting glucose (mmol/L)]/22.5. HOMA-IR values <3 means normal, 3–5 means moderate, > 5 men’s severe insulin resistance. Adiponectin was detected by a human adiponectin ELISA kit (Multi Sciences, China).
Insulin tolerance tests (ITT)
The rats were weighed, fasted 2 hours before the test. The blood was collected from the tail vein, and blood glucose was measured before the intraperitoneal injection of insulin solution (0.5 U/kg) as the first value. Blood glucose was measured at 15, 30, 45, and 60 minutes, respectively.
Glucose tolerance tests (GTT)
The rats were weighed, fasted 13 hours before the test. The blood was collected from the tail vein, and blood glucose was measured before the intraperitoneal injection of glucose solution (1 g/kg) as the first value. Blood glucose was measured at 15, 30, 45, and 60 minutes, respectively.
Cells and transfection
HEK (human embryonic kidney) 239 cell was bought from the Cell Bank of Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. The miR-21 mimic and NC mimic were synthesized by GenePharma Company (Shanghai, China). The sequence of si-TIMP3 and si-NC were designed with the BLOCK‐iT™ RNAi Designer (Invitrogen). Transfection of the miR-21 mimics, NC mimic, si-TIMP3, or si-NC was performed by ribo-FECT™ CP Transfection Kit (Ribo, Guangzhou, China) according to the manufacturer’s protocol.
Luciferase reporter assay
PCR amplified wild-type TIMP3 fragments that may contain binding sites of miR-21. Seed region mutagenesis was achieved by using a mutate reverse primer. Wild-type TIMP3 (TIMP3-WT) and mutant TIMP3 (TIMP3-MUT) fragments were inserted into the downstream of the pmirGLO promoter vector luciferase gene to generate recombinant plasmids TIMP3-WT and TIMP3-MUT, respectively. In luciferase reporter assay, recombinant plasmids were transiently co-transfected with miR-21 mimics or NC mimics, then knocked into HEK239 cells via LipofectamineTM 2000 (Life Technologies, Carlsbad, CA, USA). Twenty-four hours after transfection, luciferase activity was evaluated using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Western blotting
The primary anti-TIMP3 was bought from Abcam (Cambridge, UK). Cells with different treatments were cultured for 48 hours and lysed with RIPA lysis buffer. A BCA kit (Pierce, Rockford, IL, USA) was used to determine the total protein concentration. An equal amount of protein was isolated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked by 5% non-fat dry milk and incubated with anti-TIMP3 overnight at 4 °C. Then the membrane was washed by TBST buffer and subjected to the secondary antibody. The blot was detected by an ECL detection kit (Pierce, Rockford, IL, USA). GAPDH was used as a loading control.
Results
MiR-21 antagomir reduced the blood glucose concentration in T2DM rats
After the establishment of low dose, STZ induced T2DM rat model, the blood glucose concentration, as shown in Figure 1A. T2DM significantly increased the blood glucose concentration compared to the healthy group. Then miR-21 antagomir and negative control (NC) antagomir were intravenously injected into T2DM rats. As shown in Figure 1B, the expression of miR-21 was markedly up-regulated by T2DM and then down-regulated by miR-21 antagomir. The expression of miR-21 in T2DM rats intravenously injected with NC antagomir was still higher than in T2DM rats with miR-21 antagomir. The result of blood glucose concentration in rats after intravenous injections were like the expression level of miR-21 (Figure 1C). These results taken together indicated that miR-21 antagomir reduced the expression of miR-21 and blood glucose concentration in T2DM rats.

MiR-21 antagomir improved lipid metabolic disorder in STZ-induced T2DM rats
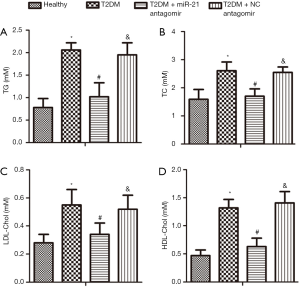
The effects of miR-21 antagomir on serum TG (Figure 2A), TC (Figure 2B), LDL-Cho (Figure 2C), and HDL-Cho (Figure 2D) in T2DM rats were tested. The results showed that T2DM increased the levels of TG, TC, LDL-Cho, and reduced the level of HDL-Cho compared to healthy rats. Then miR-21 antagomir decreased the levels of TG, TC, LDL-Cho, and restored the level of HDL-Cho in serum compared to the T1DM rats. Meanwhile, the levels of TG, TC, LDL-Cho in the NC antagomir group were higher than those in the miR-21 antagomir group while the levels were lower for HDL-Cho. In a word, miR-21 antagomir improved lipid metabolic disorder in STZ-induced T2DM rats.
MiR-21 antagomir improved insulin resistance in STZ-induced T2DM rats
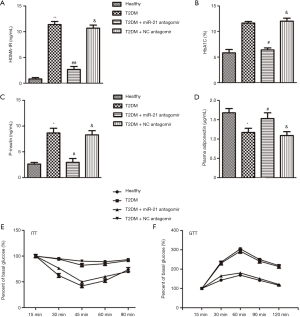
The HOMA-IR, HbA1c, plasma insulin, and plasma adiponectin were measured to determinate the glucose homeostasis. The HOMA-IR decreased in T2DM rats with miR-21 antagomir compared to the T2DM rats (Figure 3A). The HbA1c (Figure 3B) and plasma insulin (Figure 3C) were decreased in T2DM + miR-21 antagomir group compared to the T2DM group, too. As depicted in Figure 3D, the plasma adiponectin was interfered with by T2DM and restored by miR-21 antagomir. The results of percent of basal glucose after ITT and GTT were shown in Figure 3E and Figure 3F, respectively. Only healthy and T2DM + miR-21 antagomir group showed preserved insulin sensitivity, while the other two groups displayed insulin resistance. Healthy and T2DM + miR-21 antagomir groups displayed lower percent of basal glucose, showing glucose intolerance in rats in the two groups. The results showed that miR-21 improved insulin resistance in STZ-induced T2DM rats.
TIMP3 was a direct target of miR-21
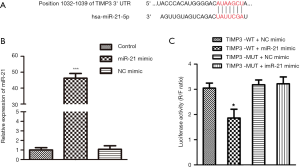
The targeted relationship between miR-21 and TIMP3 was predicted by bioinformatics (Figure 4A). HEK239 cells were used for the later experiments. The miR-21 mimic and NC mimic were transfected into HEK239 cells. The expression level of miR-21 was dramatically increased by miR-21 mimic compared to the control group (Figure 4B). The result of a luciferase activity assay was shown in Figure 4C. Compared to cells co-transfected with TIMP3-WT and NC mimic, TIMP3-MUT and miR-21 mimic, TIMP3-MUT, and NC mimic, the luciferase activity was significantly decreased in the cells co-transfected with TIMP3-WT and miR-21 mimic. This result revealed that TIMP3 was a direct target of miR-21.
MiR-21 antagomir improved insulin resistance and lipid metabolism disorder by up-regulating the expression level of TIMP3
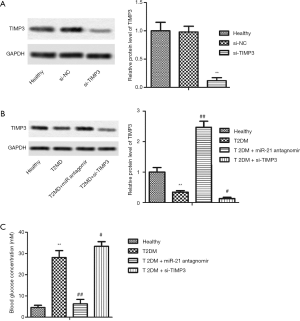
To decrease the expression of TIMP3, si-TIMP3 was intravenously injected into T2DM rats. As shown in Figure 5A, si-TIMP3 notably decreased the expression of TIMP3 compared to the si-NC group. T2DM reduced the TIMP3 level compared to the healthy group. MiR-21 antagomir extremely increased the expression of the TIMP3, while si-TIMP3 decreased the expression of TIMP3 compared to the T2DM group (Figure 5B). The blood glucose concentration in T2DM rats was down-regulated by miR-21 antagomir and up-regulated by si-TIMP3 (Figure 5C). In summary, miR-21 antagomir improved insulin resistance and lipid metabolism disorder in STZ-induced T2DM rats by up-regulating the expression level of TIMP3.
Discussion
T2DM has become a worldwide health concern. Due to its significant morbidity and mortality, the current incidence of T2DM is 9%, and it will get higher in the future (20). Current treatments cannot supply long-term effective blood glucose control. Hence, it is urgently needed to improve an effective therapeutic strategy for T2DM.
MiR-21 has been reported to be associated with the injury caused by type 2 diabetes. In T2DM with diabetic retinopathy, the expression of miR-21 was relatively increased, and it was associated with the pathogenic process of diabetic retinopathy in T2DM (21). The previous study revealed that miR-21 overexpression was a pathogenic mechanism and a potential therapeutic target for diabetic retinopathy (22). Similarly, miR-21 was a key therapeutic target for renal injury in rats with T2DM (13). In the present study, we investigated the effects of miR-21 antagomir on STZ-induced T2DM rats. In T2DM rats, the expression of miR-21 and concentration of blood glucose was significantly increased. MiR-21 antagomir in T2DM rats reduced the expression of mir-21 as well as the concentration of blood glucose. This result showed that miR-21 antagomir could reduce blood glucose concentration in T2DM rats.
Lipid metabolic disorder is a primary pathological change in T2DM. Because of its long-term complications, some experts prefer to regard T2DM as “lipocentric” instead of “glucocentric” (23). TG and cholesterol are basic components of body lipids. Dyslipidemia is characterized by elevated TG, cholesterol, and associated lipoproteins (24). TG is a commonly measured component of lipid profiles, and it is related to the incidence of T2DM (25). In previous studies, significant rises in plasma TG and TC levels in T2DM were observed (26). Consistent with earlier studies, TG, and cholesterol levels in this study were also increased in T2DM. When the LDL-Cho level is too high in the blood, the cholesterol accumulates in the body and causes metabolic disorders. Therefore, the reduction of LDL-Cho has been recognized as one of the best treatments for lipid metabolism disorders (27). HDL-Cho is related to insulin secretion (28) and blood glucose levels. An elevated level of HDL-Cho could reduce the blood glucose level and down-regulation of HDL-Cho could increase the blood glucose level (29). In the present study, miR-21 antagomir down-regulated the contents of TG, TC, LDL-Cho, and up-regulated HDL-Cho. In summary, miR-21 antagomir improved lipid metabolic disorder in STZ-induced T2DM rats.
Insulin resistance is an early feature of the T2DM condition. Insulin resistance has been identified as an important risk factor for T2DM and has been reported to be associated with increased adverse events in T2DM patients (16). HOMA-IR was developed by Matthews et al. and has been widely used for the evaluation of insulin resistance (30). High HOMA-IR suggests low insulin sensitivity. Hyperglycemia, hyperinsulinemia, and increased glycation of hemoglobin are important symptoms of T2DM. So the test of HbA1c is a common diagnostic tool for T2DM management and research (31). Adiponectin is an adipose tissue-derived hormone, known to have insulin-sensitizing (32). It has been reported to decrease insulin resistance (33). ITT evaluates insulin sensitivity by monitoring fasting plasma glucose disappearance over time in response to an injection of insulin (34). GTT evaluates glucose tolerance by detecting the plasmatic glucose of the body of an injected glucose load (35). In the present study, miR-21 antagomir decreased the HOMA-IR, HbA1c, P-insulin, and increased the adiponectin in T2DM rats. Moreover, ITT and GTT results suggested higher insulin sensitivity and glucose tolerance regulated by miR-21 antagomir. In conclusion, miR-21 antagomir improved insulin resistance in STZ-induced T2DM rats.
Bioinformatics predicted the targeting relationship between TIMP3 and miR-21. TIMP are soluble proteins that interact with the protease to regulate the activity of metalloproteinases (36). TIMP3 is a unique member of the TIMP family and has been proved to inhibit the activity of various proteases and receptors (37). The relationship between miR-21 and TIMP3 has been revealed in several studies. In cumulus cells, miR-21 promotes cumulus expansion and oocyte maturation through down-regulating TIMP3 (38). In the ischemic retina, activation of miR-21 is involved in the down-regulation of TIMP3 (39). Consistent with the reports mentioned above, the result of the luciferase activity assay showed that TIMP3 was a direct target of miR-21 in T2DM rats, too.
TIMP3 is associated with pathological mechanisms in T2DM. It has been reported that TIMP3 was down-regulated in T2DM, and deficiency of TIMP3 was involved with insulin resistance and vascular inflammation (40). In atherosclerotic plaques subjects with T2DM, a decrease of TIMP3 expression increased the activity of inflammatory (41), which may promote atherothrombosis. MiR-21 played a key role in the development of tumors by different molecular mechanisms (42,43). In renal cancer, the carcinogenesis of miR-21 was found to be related to TIMP3 (44). In this study, TIMP3 was decreased in T2DM rats compared to the healthy rats. Si-TIMP3 increased blood glucose in T2DM rats compared to regular T2DM rats. Furthermore, miR-21 antagomir extremely increased the expression of TIMP3 while notably decreased the blood glucose concentration in T2DM rats. These pieces of evidence showed that miR-21 antagomir improved insulin resistance and lipid metabolism disorder by up-regulating the expression level of TIMP3.
In summary, we showed that miR-21 antagomir improved insulin resistance and lipid metabolism disorder in STZ-induced T2DM rats by up-regulating the expression level of TIMP3. All the results in the present study suggested that miR-21 antagomir can be used as an effective therapeutic strategy for STZ-induced T2DM. However, further study needs to be done to investigate the effect of miR-21 antagomir on clinical application further.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Experimental Animal Ethics Committee of People’s Hospital of Dujiangyan, The People’s Hospital of Dujiangyan, ratified all the animal experiments in this study (SYXK2018-213). Procedures and protocols were performed in strict accordance to the Guide for the Care and Use of Laboratory Animals of the International Association for the Study of Pain (IASP).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88-98. [Crossref] [PubMed]
- Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol 2017. [Crossref] [PubMed]
- Mondal P, Prasad A, Girdhar K. Interventions to improve β-cell mass and function. Ann Endocrinol (Paris) 2017;78:469-77. [Crossref] [PubMed]
- Prasad RB, Groop L. The Genetic Architecture of Type 2 Diabetes. John Wiley & Sons, Ltd.; 2017.
- Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544-50. [Crossref] [PubMed]
- Wu YH, Li JY, Wang C, et al. The ACE2 G8790A Polymorphism: Involvement in Type 2 Diabetes Mellitus Combined with Cerebral Stroke. J Clin Lab Anal 2017;31. [Crossref] [PubMed]
- Landau Z, Raz I, Wainstein J, et al. The role of insulin pump therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev 2017. [Crossref] [PubMed]
- Wu H, Ng R, Chen X, et al. MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut 2016;65:1850-60. [Crossref] [PubMed]
- An F, Liu Y, Hu Y. miR-21 inhibition of LATS1 promotes proliferation and metastasis of renal cancer cells and tumor stem cell phenotype. Oncol Lett 2017;14:4684-8. [Crossref] [PubMed]
- Haghpanah V, Fallah P, Tavakoli R, et al. Antisense-miR-21 enhances differentiation/apoptosis and reduces cancer stemness state on anaplastic thyroid cancer. Tumour Biol 2016;37:1299-308. [Crossref] [PubMed]
- Li ZB, Li ZZ, Li L, et al. MiR-21 and miR-183 can simultaneously target SOCS6 and modulate growth and invasion of hepatocellular carcinoma (HCC) cells. Eur Rev Med Pharmacol Sci 2015;19:3208-17. [PubMed]
- Mastropasqua R, Toto L, Cipollone F, et al. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res 2014;43:92-107. [Crossref] [PubMed]
- Zhong X, Chung AC, Chen HY, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 2013;56:663-74. [Crossref] [PubMed]
- Haghani K, Asadi P, Taheripak G, et al. Association of mitochondrial dysfunction and lipid metabolism with type 2 diabetes mellitus: A review of literature. Frontiers in Biology 2018;13:406-17. [Crossref]
- Giannarelli R, Aragona M, Coppelli A, et al. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 2003;29:6S28-35.
- Hsu CH. Different impacts of metabolic syndrome components on insulin resistance in type 2 diabetes. Int J Endocrinol 2013;2013:740419. [Crossref] [PubMed]
- Gogoi B, Chatterjee P, Mukherjee S, et al. A polyphenol rescues lipid induced insulin resistance in skeletal muscle cells and adipocytes. Biochem Biophys Res Commun 2014;452:382-8. [Crossref] [PubMed]
- Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 2015;4:R1-15. [Crossref] [PubMed]
- Deng R, Yang D, Radke A, et al. The hypolipidemic agent guggulsterone regulates the expression of human bile salt export pump: dominance of transactivation over farsenoid X receptor-mediated antagonism. J Pharmacol Exp Ther 2007;320:1153-62. [Crossref] [PubMed]
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271-81. [Crossref] [PubMed]
- Jiang Q, Lyu X-M, Yuan Y, et al. Plasma miR-21 expression: an indicator for the severity of Type 2 diabetes with diabetic retinopathy. Bioscience Reports 2017. [Crossref] [PubMed]
- Chen Q, Qiu F, Zhou K, et al. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARalpha. Diabetes 2017;66:1671-82. [Crossref] [PubMed]
- Matthijs KCH, Marco M, Patrick S. Lipotoxicity and Mitochondrial Dysfunction in Type 2 Diabetes. Immunology, Endocrine & Metabolic Agents in Medicinal Chemistry (Under Re-organization) 2007;7:3-17.
- Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran 2015;29:240. [PubMed]
- Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016;39:833-46. [Crossref] [PubMed]
- Aierken A, Buchholz T, Chen C, et al. Hypoglycemic effect of hawthorn in type II diabetes mellitus rat model. J Sci Food Agric 2017;97:4557-61. [Crossref] [PubMed]
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. Lancet 2002;360:7-22. [Crossref] [PubMed]
- Fryirs MA, Barter PJ, Appavoo M, et al. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol 2010;30:1642-8. [Crossref] [PubMed]
- Song X, Teng J, Wang A, et al. Positive correlation between serum IGF-1 and HDL-C in type 2 diabetes mellitus. Diabetes Res Clin Pract 2016;118:44-9. [Crossref] [PubMed]
- Nolan JJ, Faerch K. Estimating insulin sensitivity and beta cell function: perspectives from the modern pandemics of obesity and type 2 diabetes. Diabetologia 2012;55:2863-7. [Crossref] [PubMed]
- Hirst JA, McLellan JH, Price CP, et al. Performance of point-of-care HbA1c test devices: implications for use in clinical practice - a systematic review and meta-analysis. Clin Chem Lab Med 2017;55:167-80. [Crossref] [PubMed]
- Bi X, Ai H, Wu Q, et al. Insulin Resistance Is Associated with Interleukin 1beta (IL-1beta) in Non-Diabetic Hemodialysis Patients. Med Sci Monit 2018;24:897-902. [Crossref] [PubMed]
- Sirbu AE, Buburuzan L, Kevorkian S, et al. Adiponectin expression in visceral adiposity is an important determinant of insulin resistance in morbid obesity. Endokrynologia Polska 2018;69:252. [Crossref] [PubMed]
- Vinue A, Gonzalez-Navarro H. Glucose and Insulin Tolerance Tests in the Mouse. Methods Mol Biol 2015;1339:247-54. [Crossref] [PubMed]
- Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010;3:525-34. [Crossref] [PubMed]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 2010;1803:55-71. [Crossref] [PubMed]
- Stohr R, Cavalera M, Menini S, et al. Loss of TIMP3 exacerbates atherosclerosis in ApoE null mice. Atherosclerosis 2014;235:438-43. [Crossref] [PubMed]
- Bo P, Julang L. MicroRNA-21 up-regulates metalloprotease by down-regulating TIMP3 during cumulus cell-oocyte complex in vitro maturation. Mol Cell Endocrinol 2018;477:29-38. [Crossref] [PubMed]
- Gutsaeva DR, Thounaojam M, Rajpurohit S, et al. STAT3-mediated activation of miR-21 is involved in down-regulation of TIMP3 and neovascularization in the ischemic retina. Oncotarget 2017;8:103568-80. [Crossref] [PubMed]
- Menghini R, Menini S, Amoruso R, et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 2009;136:663-72.e4. [Crossref] [PubMed]
- Park S, Kim K, Bae IH, et al. TIMP3 is a CLOCK-dependent diurnal gene that inhibits the expression of UVB-induced inflammatory cytokines in human keratinocytes. FASEB J 2018;32:1510-23. [Crossref] [PubMed]
- Xu S, Shi L. High expression of miR-155 and miR-21 in the recurrence or metastasis of non-small cell lung cancer. Oncol Lett 2019;18:758-63. [PubMed]
- Wang H, Tan Z, Hu H, et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019;19:738. [Crossref] [PubMed]
- Chen J, Gu Y, Shen W. MicroRNA-21 functions as an oncogene and promotes cell proliferation and invasion via TIMP3 in renal cancer. Eur Rev Med Pharmacol Sci 2017;21:4566-76. [PubMed]


