
Quietness Circ 0000962 promoted nerve cell inflammation through PIK3CA/Akt/NF-κB signaling by miR-302b-3p in spinal cord injury
Introduction
Intraspinal nerve block is a kind of anesthesia method that is often used clinically, with the advantages of safety and less equipment. It is also characterized with simple operation, small medical expense, better effect of analgesia and muscle relaxation, controllable characteristics as well as the maintenance of the patient’s consciousness (1). However, it also has shortcomings, such as hypotension, tachycardia, headache after puncture, damage to blood vessels and nerves, spinal cord injury (SCI) and so on (2). Although the incidence of serious complications is relatively low, it is more difficult to recover neurological function in the event of severe neurological complications (3). Recently, due to the reports on the occurrence of neurological complications caused by the spinal canal block after local anesthesia, intraspinal block has gained more attention (4).
A large number of studies have shown that SCI can cause the release of local inflammatory cytokine, which may be associated with neurological dysfunction (5). In order to reduce the direct SCI caused by puncture, many scholars have concentrated on the research and development on the lumbar puncture needle for many years, making improvement from the tip shape and needle diameter size respectively (6). The growth cone is the end of the neuronal axon, which plays an important role in the growth of nerve cells (5). Therefore, the study of the growth cone can provide good observation of the effects of some external stimuli or external substances on the development of nerve tissue (7).
For more than 30 years, Circular RNA served a crucial role in the occurrence and development of human diseases and had been acknowledged as products of aberrant splicing, gene rearrangement, or non-linear reverse splicing (8). MicroRNAs are 19–24 nucleotides in length, small noncoding RNAs, which posttranscriptionally regulate gene expression by targeting the 3’untranslated region (3’-UTR) of target mRNAs (9,10). With the profound research on miRNA, these small molecules are found to play important roles in the pathophysiology of the cardiovascular system, including arrhythmias, cardiac hypertrophy, fibrotic heart failure and angiogenesis (11). Therefore, the aim of this study was to analyze the effects of Circ 0000962 attenuates toxicity in SCI and its possible mechanism.
Methods
Animals
Forty Sprague-Dawley rats (6 weeks old, male) supplied by Animal Experimental Center of Medicine, University were randomly distributed into two groups: the sham group, SCI model group. Briefly, in SCI model group, the rats were fixed in the prone position and anesthetized by 35 mg/kg of pentobarbital. Skin was cut to 2–3 cm middle incision in the back, and vertebral T7–T9 were exposed. After stabilizing vertebral T7 and T9, a laminectomy was performed at the thoracic level T8. A syringe needle was used to induce the injury, which was released from a height of 12.5 mm above the surface of the cord, inflicting a moderate contusion. Hemostatic suture was performed layer by layer, and alcohol was then applied for disinfection. Three days after the operation, the rats received an intramuscular injection of l g gentamicin to prevent infection. The stitches were taken out after one week (Figure 1). Rats in the sham group were performed with laminectomy of vertebral T8 without injury. This study was approved by the Animal Experiment Committee of the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China.
Basso, Beattie, and Bresnahan (BBB) locomotor rating score and water content of SCI
BBB locomotor rating score was performed after treatment with Circ 0000962. BBB locomotor rating score was scored in an open field according to the locomotor rating scale of 0 (complete paralysis) to 21 (normal locomotion). After treatment with Circ 0000962, rat was sacrificed under 35 mg/kg of pentobarbital and Spinal cord tissue was weighed as wet weighing. Spinal cord tissue was dryed at 70 °C for 72 h and weighed as drying weighing. Water content of SCI was calculated using wet weighing (g)/drying weighing (g) × 100%.
Cell culture
Nerve cell lines AGE1.HN cell were cultured in DMEM medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) at 37 °C in a humidified atmosphere with 5% CO2. Circ 0000962, anti-Circ 0000962 and negative mimics were transfected into cell using Lipofectamine 2000 (Thermo Fisher Scientific, Inc. Waltham, MA, USA). After transfection at 48 h, cell was treated with LPS (100 ng/mL) for 4 h.
Measurement of inflammation and caspase-8/9 activity
Serum of rats or cell samples were collected after 2,000 g for 10 min at 4 °C, and was used to measure TNF-α, IL-1β, IL-6 and IL-18 levels using ELISA KITS. Cell was treated for 6 h, supernatants of cell were collected after 2,000 g for 10 min at 4 °C, and was used to measure TNF-α, IL-1β, IL-6 and IL-18 levels using ELISA KITS. Absorbance was measured using ELISA reader at 450 nm.
Western blot analysis
Tissue samples or cell samples were homogenated or lysed using RIPA assay and protein content was measured using BCA assay. And 50 µg proteins was then subjected to 8–12% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Merck KGaA, Darmstadt, Germany). The membrane was blocked in 5% non-fat milk in Tris-buffered saline/Tween 20 (TBST) for 1 h, and subsequently incubated with NF-κB, PI3K, p-Akt and GAPDH (Cell Signaling Technologies, Danvers, MA, USA) at 4 °C overnight. The membrane was washed with TBST and then incubated with horseradish peroxidase labeled goat anti-rat secondary antibody (1:1,000; Cell Signaling Technologies, Danvers, MA, USA) at 37 °C for 1 h. Protein blank was detected with enhanced chemiluminescence (ECL) assay.
Statistical analysis
Data are expressed as mean ± SD. Student’s t-test or ANOVA at P<0.05 was considered statistically significant. P<0.05 was considered to indicate a statistically significant difference.
Results
Circ 0000962 in inflammation in SCI model
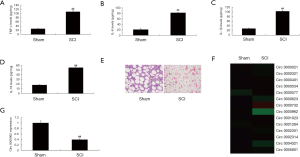
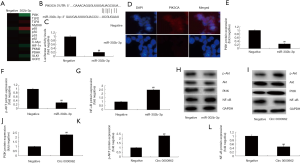
Circ 0000962 was firstly investigated in our lab, therefore, we explored the function and mechanism of miRNA-203a in SCI model. In model of rats, TNF-α, IL-1β, IL-6 and IL-18 levels were increased in SCI modal rat, compared with sham group (Figure 2A,B,C,D). HE staining showed that the number of Spinal cord cells in SCI model group was lower than that of sham group (Figure 2E). We found that Circ 0000962 expression was decreased in SCI model rat, compared with sham group (Figure 2F,G).
Circ 0000962 regulates inflammation in vitro model of SCI
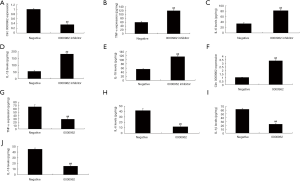
To analyze the function of Circ 0000962 on inflammation in SCI model, Circ 0000962 or anti-Circ 0000962 mimics regulates Circ 0000962 expression in vitro model. As shown in Figure 3A, Circ 0000962 inhibitor decreased the expression of Circ 0000962 in vitro model, compared with negative mimics group. Down-regulation of Circ 0000962 increased TNF-α, IL-1β, IL-6 and IL-18 levels in vitro model, compared with negative group (Figure 3B,C,D,E). Then, 0000962 mimics increased the expression of Circ 0000962 expression in vitro model, compared with negative group (Figure 3F). Over-expression of Circ 0000962 reduced TNF-α, IL-1β, IL-6 and IL-18 levels in vitro model, compared with negative group (Figure 3G,H,I,J).
Circ 0000962 regulates inflammation in vitro model of SCI by miR-302b-3p
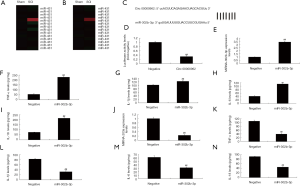
To explain the mechanism of Circ 0000962 on inflammation in vitro model of SCI by miR-302b-3p. MiR-302b-3p expression was increased in SCI model, compared with sham group (Figure 4A,B,C). Then, over-expression of Circ 0000962 promoted miR-302b-3p expression in vitro model of SCI, compared with negative group (Figure 4D). 3’UTR region of Circ 0000962showed potential alignment with miR-302b-3p sequence and luciferase reporter activity levels were reduced by over-expression of Circ 0000962, compared with negative group. There was increase the expression of miR-302b-3p in over-expression of miR-302b-3p, compared with negative group (Figure 4E). Over-expression of miR-302b-3p increased TNF-α, IL-1β, IL-6 and IL-18 levels, compared with negative group (Figure 4F,G,H,I). Then, anti-miR-302b-3p mimics reduced the expression of miR-302b-3p, and inhibited TNF-α, IL-1β, IL-6 and IL-18 levels, compared with negative group (Figure 4J,K,L,M,N).
MiR-302b-3p regulates inflammation in vitro model of SCI via PI3K/Akt/NF-κB signaling
To confirm the mechanism of miR-302b-3p on inflammation in vitro model of SCI, the signaling was measured using gene chip. Gene chip showed that Over-expression of miR-302b-3p increased p65 and reduced PI3K expression in vitro model, compared with negative group (Figure 5A). Figure 5B,C showed that 3’UTR region of PIK3CA showed potential alignment with miR-302b-3p sequence and luciferase reporter activity levels were reduced by over-expression of miR-302b-3p, compared with negative group. Over-expression of miR-302b-3p suppressed PIK3CA expression in vitro model, compared with negative group (Figure 5D). As shown in Figure 5E,F,G,H, PI3K and p-Akt protein expressions were reduced, and NF-κB expression was increased in vitro model by over-expression of Circ 0000962, compared with negative mimics group. Over-expression of Circ 0000962 induced PI3K and p-Akt protein expressions and suppressed NF-κB protein expressions in vitro model, compared with negative mimics group (Figure 5I,J,K,L).
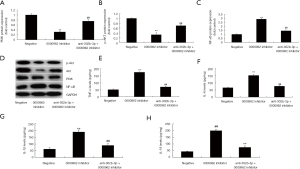
MiR-302b-3p reduced the effects of circ 0000962 on inflammation in vitro model of SCI through PI3K/Akt/NF-κB signaling
To analyze the role of miR-302b-3p in the effects of circ 0000962 on inflammation in vitro model of SCI, miR-302b-3p mimcs increased miR-302b-3p expression, induced PI3K and p-Akt protein expressions and suppressed NF-κB protein expressions in vitro model following over-expression of Circ 0000962, compared with Experimental Cell Research of Circ 0000962 group (Figure 6A,B,C,D). The activation of miR-302b-3p reduced the effects of circ 0000962 on inflammation (TNF-α, IL-1β, IL-6 and IL-18 levels) in vitro model of SCI, compared with Experimental Cell Research of Circ 0000962 group (Figure 6E,F,G,H).
Discussion
In recent years, various treatments have focused on axonal and dendritic repair to enhance recovery from CNS injury. SCI is medically and socioeconomically enervating and lacking in effective therapies. Improvements in the neurosciences have drawn more attention to SCI research, and until now, no available treatments for SCI could effectively decrease or stop the disease progression. The elucidation of the pathophysiological mechanisms underlying SCI is necessary for studying effective therapies for SCI. The development of SCI pathology is guided by the two leading mechanisms of damage, which are primary injury and secondary injury (4). Primary injury occurs passively within a short period of time after injury, resulting in irreversible damage, while secondary injury is reversible, controllable and complicated with self-destructive cascade reaction process, which is the main reason for aggravating neurological dysfunction (12). The research on miRNAs may provide new insights for the study of the molecular mechanisms of SCI. In this study, we identified that circ 0000962 expression was decreased in SCI model rat.
It was shown that high expression of inflammatory cytokines after SCI would increase the degree of secondary puncture injury, as the inflammatory cells, especially neutrophils aggregate to the injured site after injury, and neutrophils could release elastase and reactive oxygen free radicals, which further destructed vascular endothelial integrity, increased tissue edema and necrosis, so that neurological deterioration of inflammatory cytokines could also cause neurons and oligodendrocyte apoptosis through a pathway to stimulate the proliferation of astrocytes, leading to local formation of glial paralysis, inhibition of axonal regeneration, upregulation of other inflammatory-related gene expression (6,13). A wide range of cytokines can be divided into inflammatory cytokines and anti-inflammatory cytokines according to the differences in inflammatory response (13). A variety of proinflammatory cytokines and chemokines are significantly increased in post-injury area, therefore, the post-inflammatory cascade is expanded to increase secondary damage to SCI (5). We found revealed that over-expression of Circ 0000962 decreased TNF-α, IL-1β, IL-6 and IL-18 levels in vitro model. miR-302b-3p expression was increased in SCI model. Wang et al. reported that knockdown miR-302b reduced LPS-induced injury by in C28/I2 chondrocytic cells (14), which was also in accordance with our results.
Many studies have indicated that the inhibition of NF-κB activation protects the spinal cord in SCI (15-17). Thus, NF-κB activation is the key element of the secondary neuronal damage in SCI. In the present study, PI3K and p-Akt protein expressions were increased, and NF-κB expression was reduced in vitro model by over-expression of Circ 0000962. NF-κB activity as well as TNF-a, IL-1b, and IL-6 expression was suppressed by Circ 0000962 treatment, indicating that NF-κB activation contributes to increased levels of these cytokines. Previous results and data showing the severity of neuronal injury were dependent on the expression of cytokines after a SCI (18).
PI3K/Akt signaling pathway plays an important role in neurophysiological pathology as a central controller for cell growth, proliferation, survival and differentiation under conditions such as extracellular signal, growth factor, cell energy status and emergency response (19,20). PI3K/Akt signaling pathway has been involved in the process of glial scar formation, and attenuation of PI3K/Akt pathway may beneficially inhibit glial scar formation after SCI. Guo et al. indicated the microRNA-302b-3p suppresses cell proliferation through AKT pathway in gastric cancer (21). Our results revealed that miR-302b-3p reduced the effects of circ 0000962 on inflammation in vitro model of SCI through PI3K/Akt/NF-κB signaling.
In conclusion, our study revealed that Circ 0000962 weakened inflammation of SCI through anti-inflammation effects by PI3K/Akt/NF-κB-dependent signaling in SCI by miR-302b-3p. This study contributes to our understanding of molecular mechanisms of Circ 0000962, and the investigation on the new molecular therapeutic targets in SCI.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Animal Experiment Committee of the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (No. 201711(16)).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kornblith LZ, Kutcher ME, Callcut RA, et al. Mechanical ventilation weaning and extubation after spinal cord injury: a Western Trauma Association multicenter study. J Trauma Acute Care Surg 2013;75:1060-9; discussion 1069-70. [Crossref] [PubMed]
- Mohammadi V, Khalili M, Eghtesadi S, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: a clinical trial. Spinal Cord 2015;53:621-4. [Crossref] [PubMed]
- Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine 2012;17:151-6. [Crossref] [PubMed]
- Arora M, Harvey LA, Glinsky JV, et al. Telephone-based management of pressure ulcers in people with spinal cord injury in low- and middle-income countries: a randomised controlled trial. Spinal Cord 2017;55:141-7. [Crossref] [PubMed]
- Allison DJ, Josse AR, Gabriel DA, et al. Targeting inflammation to influence cognitive function following spinal cord injury: a randomized clinical trial. Spinal Cord 2017;55:26-32. [Crossref] [PubMed]
- Allison DJ, Ditor DS. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J Neuroinflammation 2015;12:204. [Crossref] [PubMed]
- Norouzi Javidan A, Sabour H, Latifi S, et al. Does consumption of polyunsaturated fatty acids influence on neurorehabilitation in traumatic spinal cord-injured individuals? A double-blinded clinical trial. Spinal Cord 2014;52:378-82. [Crossref] [PubMed]
- Xu Y, Yao Y, Liu Y, et al. Elevation of circular RNA circ_0005230 facilitates cell growth and metastasis via sponging miR-1238 and miR-1299 in cholangiocarcinoma. Aging (Albany NY) 2019;11:1907-17. [Crossref] [PubMed]
- Rom S, Dykstra H, Zuluaga-Ramirez V, et al. miR-98 and let-7g* protect the blood-brain barrier under neuroinflammatory conditions. J Cereb Blood Flow Metab 2015;35:1957-65. [Crossref] [PubMed]
- Ji ML, Lu J, Shi PL, et al. Dysregulated miR-98 Contributes to Extracellular Matrix Degradation by Targeting IL-6/STAT3 Signaling Pathway in Human Intervertebral Disc Degeneration. J Bone Miner Res 2016;31:900-9. [Crossref] [PubMed]
- Sun C, Liu H, Guo J, et al. MicroRNA-98 negatively regulates myocardial infarction-induced apoptosis by down-regulating Fas and caspase-3. Sci Rep 2017;7:7460. [Crossref] [PubMed]
- Olsen U, Brox JI, Bjork IT. Preoperative bowel preparation versus no preparation before spinal surgery: A randomised clinical trial. Int J Orthop Trauma Nurs 2016;23:3-13. [Crossref] [PubMed]
- Kwon BK, Stammers AM, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2010;27:669-82. [Crossref] [PubMed]
- Wang Y, Yu T, Jin H, et al. Knockdown MiR-302b Alleviates LPS-Induced Injury by Targeting Smad3 in C28/I2 Chondrocytic Cells. Cell Physiol Biochem 2018;45:733-43. [Crossref] [PubMed]
- Shi C, Zhang X, Li X, et al. Effects of microRNA-21 on the biological functions of T-cell acute lymphoblastic lymphoma/leukemia. Oncol Lett 2016;12:4173-80. [Crossref] [PubMed]
- Goldshmit Y, Frisca F, Pinto AR, et al. Fgf2 improves functional recovery decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav 2014;4:187-200. [Crossref] [PubMed]
- Wei HY, Ma X. Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-jB pathway after spinal cord injury in rats. Neurol Sci 2014;35:1763-8. [Crossref] [PubMed]
- Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia 2008;56:259-70. [Crossref] [PubMed]
- Liu S, Yang Y, Jin M, et al. Xenon-delayed postconditioning attenuates spinal cord ischemia/reperfusion injury through activation AKT and ERK signaling pathways in rats. J Neurol Sci 2016;368:277-84. [Crossref] [PubMed]
- Zhu H, Xie R, Liu X, et al. MicroRNA-494 improves functional recovery and inhibits apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal cord injury. Biomed Pharmacother 2017;92:879-87. [Crossref] [PubMed]
- Guo B, Zhao Z, Wang Z, et al. MicroRNA-302b-3p Suppresses Cell Proliferation Through AKT Pathway by Targeting IGF-1R in Human Gastric Cancer. Cell Physiol Biochem 2017;42:1701-11. [Crossref] [PubMed]


