Prospective evaluation of the quality of life of oral tongue cancer patients before and after the treatment
Introduction
In India, head and neck cancers presents a major public health problem. Of these, oral tongue squamous cell carcinoma is one of the most common malignancies among the males (1).
The tongue is one of the most important structures in the oropharyngeal deglutition process: performing functions of intraoral food manipulation, bolus preparation, bolus ejection to the oesophagus and in the speech production process.
Patients with tongue cancers not only have to face a life threatening disease, but have to deal with the impact of the disease and the resulting surgical intervention on their quality of life (QOL) as well. Therefore, health related QOL has been increasingly thought to be of paramount importance in the assessment of treatment results of oral tongue squamous cell carcinoma. Aesthetic and functional sequel due to surgical incision and cancer resection, often associated with post-operative radiotherapy (RT), always modify the patient’s self-perception and ability to interact with others in daily social life (2).
Health related QOL is increasingly being recognised as an important issue in oncology. It is a multidimensional concept which comprises four core domains: (I) physical functioning; (II) psychological functioning; (III) social interaction; and (IV) disease- and treatment-related symptoms (3).
QOL is an important indicator of treatment outcome and is used increasingly as an end point in clinical trials. Patients with head and neck cancer are prone to psychological problems because social interaction and emotional expression depend to a great extent upon the structural and functional integrity of the head and neck region. This is particularly true for cancer of the oral cavity and oropharynx. Not only do these patients have to face a life threatening disease, but they also have to deal with the impact of both the disease and its treatment on appearance and important functions like eating, swallowing and speaking. This may lead to decreased physical and role functioning and to problems with communication, social interaction (4). Depression occurs frequently in these patients (5-7).
QOL measures an individual’s sense of his place within the cultural and intellectual conditions in which he lives and takes into account his expectations and worries; it defines what is most important for each individual. QOL studies provide physicians with information about the impact of the disease, the treatment of symptoms and the side effects (8).
There are several head and neck disease-specific QOL questionnaires devised to measure the specific consequences of such disease and its treatment on the affected patients, such as the University of Washington Quality of life Questionnaire (UW-QOL) (9), Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N), and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Head and Neck module (EORTC-H&N35) (10). All of these comprise different domains, including domains of mental and social functions as well as specific symptoms such as swallowing and speech. We used the UW-QOL (9) because of its wider acceptability and reliability.
In the developing countries like India till now there is a strong fear about the cancer treatment and its grave consequences on the QOL after the treatment and till now there are very few studies which have studied the QOL after the tongue cancer treatment but all of these had less sample size and no QOL comparison before and after the treatment. There is a need for systematic prospective research in this field using standardised and validated instruments.
Material and methods
This prospective study was to evaluate the changes in QOL after 12 months post treatment from their pre-treatment levels, using specific questionnaires of well-known acceptability, responsiveness, and validity, with special emphasis on domains such as chewing, swallowing, speech, psychological aspects (mood and anxiety) and pain.
This prospective analytical study included a total of 72 patients affected by oral tongue cancer (T1-2N0M0) who underwent treatment (wide local excision/hemiglossectomy with selective/functional neck dissection + RT) from 2009 to 2012 at the Department of Oncosurgery and Department of Otorhinolaryngology-Head and Neck Surgery, Sir Ganga Ram Hospital, New Delhi. Patients were followed up to 12 months post treatment. The lesion only involved the oral tongue and no clinically palpable neck node and without any distant metastasis (PET scan was done to rule out any distant and nodal metastasis).
Diagnosed cases of early oral tongue squamous cell carcinoma with stage T1-2N0M0, who were previously untreated, were included in the study.
Patients with a history of any comorbidity like brain stroke or other neurologic impairment that could lead to cognitive deficit, any psychiatric problem, major cardiovascular problems, patients with tumour recurrence, lost to follow up, patients who still using tobacco, smoking and alcohol and patients who refused to participate in the study were excluded from the study.
Patient information was collected at diagnosis and before onset of therapy and it included sociodemographic data (age, gender, schooling, employment status and marital status), comorbidity, diagnosis (tumour location, size and staging) and QOL. Comorbidity was assessed by studying the patient’s clinical history, cardiovascular, respiratory, neurological, psychiatric history and tobacco/alcohol consumption.
Informed consent was taken from every patient and institutional ethical clearance was taken.
We comparatively evaluated the QOL of the oral tongue cancer patients before and after the treatment. All the patients underwent for RT after the definitive surgery (wide local excision + selective neck dissection/functional neck dissection). QOL questionnaires based on UW-QOL (9) proforma was given to every patient before starting the treatment and one year after the completion of treatment. UW-QOL questionnaires contain 12 domains which were: pain, appearance, activity, recreation, speech, chewing, swallowing, shoulder pain, taste, saliva, mood and anxiety. Responses were calculated as scores, from 0 (poor health) to 100 (excellent health). The questionnaires were administered by the investigator.
Statistical analysis
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. Continuous variables were presented as mean ± standard deviation (SD), and categorical variables are presented as frequency and percentage. The comparison of normally distributed continuous variables from pre-treatment to post treatment was done using Paired t-test. P<0.05 was considered statistically significant.
Results and analysis
Out of total 72 patients who were treated for tongue carcinoma from 2009 to 2012 at our tertiary care centre, only 39 (54.2%) patients were eligible for the study. Death occurred in 12 patients, 4 patients had disease recurrence and 17 patients lost to follow up.
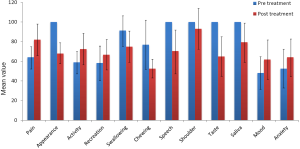
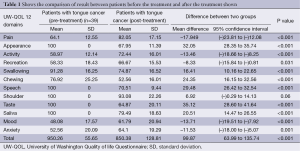
In total 39 patients who participated in the study, the mean age ± SD was 51.62±12.23 years. Thirty four male and five female patients were assessed; wide local excision of the tongue with selective neck dissection were performed in 16 patients on left side and 23 on right side. All 39 patients received postoperative RT (Table 1, Figure 1).
Full table
In our study we evaluated the QOL score changes after the treatment in tongue cancer patients, overall QOL became poor but in five domains it was improved. As the above table shows that there was significant improvement in pain scores, overall activity of the patient was significantly improved, recreational activities were also significantly improved and mental status of patient which included the mood and anxiety level were also significantly improved.
In seven domains which included the appearance of the patient, swallowing, chewing, speech, shoulder pain and discomfort, taste and saliva production scores were significantly worsen.
Discussion
Cancer in our society, especially in developing countries like India is still considered as a very grave disease in terms of its impact over the QOL after the treatment but our study shows that physical and psychosocial domains which are considered very important for the QOL became improved after the treatment.
Physical domains
We included the pain, chewing, swallowing, taste, saliva production, recreation, speech, physical appearance, overall activity and shoulder pain. Pain at the surgical site was improved in overall patients postoperatively. Chewing of food was not improved; even it became worse as the patient was able to take solid foods preoperatively but after the treatment patients were not able to take the solid foods and could only took semisolids and liquids. Swallowing, saliva production and the taste became worsen in most of the cases mainly due to the effect of RT. Speech of patients was also become worsen due to the combined effect of surgery and the RT. Speech production of the patients became worsen due the effect of surgery and RT but most of the patient’s speech was understandable by their friends and relatives. Physical appearance was changed mainly due to the effect of the RT and it was mainly because of the skin charring. Overall activities of the patients became improved as before the treatment patients were not able do their routine job duties but after the treatment patients activities were improved, on asking it was found that, this lack in activities was due to the combined effect of mental stress due cancer and pain which was improved after the treatment. Shoulder pain was mild and developed in few patients due to selective or functional neck dissection, though spinal accessory nerve was preserved in every case and it may be due the stretching of the nerve and scar hypertrophy which was gradually improving. Most of the physical domains were gradually improving so QOL of the patients was also gradually improving. The pattern of a physical deterioration after treatment has been described by other authors’ also (11-13). In all these studies there was the deterioration of the physical domains as these studies included the large composite oral and oropharyngeal resections combined with the RT. In a small prospective study in patients with oropharyngeal carcinoma treatment was associated with worsening of QOL after 12 months, in particular with regard to chewing, swallowing and shoulder disability (14). In another study the extent of surgery for oral tongue cancer was found to be correlated with functional limitations (11).
Lazarus et al. (15) compared the tongue strength in patients after the surgery and after the combined therapy (surgery and RT) and found tongue strength to be lower in the surgical group that also underwent postoperative RT in terms of speech, chewing and swallowing similarly in our study all patients received postoperative RT and speech, swallowing and chewing was worsen. Lopez-Jornet et al. (16) in their cross sectional study also concluded that the patients who received surgery combined with RT or chemotherapy showed worse score and a significant reduction to QOL.
Psychosocial domains
These included recreational activities, mood of the patients and anxiety levels of the patients. From the literature there appears a pattern of treatment leading to long term physical morbidity (damaged appearance, problems with taste, chewing problems, eating, swallowing and speech; dry mouth, sticky saliva) with important potential implications for psychosocial functioning. In American study patients with Head and Neck cancer scored worse both before and after treatment on the mental health component summary score of the SF-36 compared to United States population norms (13). In our study mental health was improved after the 12 months of treatment in terms of their anxiety and depression alleviation. However, in a retrospective study in patients 6 months to 21 years after treatment for Head and Neck Cancer it was suggested that in the long run there is deterioration in psychosocial functioning (17). In a Spanish study by Infante-Cossio et al. (8), revealed that the emotional scale shows the greatest alterations followed by the physical scale with slightly better scores and they categorised their results according to age, gender and site of tumour, in the present study psychological domains shows better results with improvement in patients anxiety and depression levels after the treatment and also improvement in some important physical domains but there was no significant difference according to variable like gender and age.
Depression and Anxiety has been shown to occur frequently in patients with oral and oropharyngeal cancer (5-7). In a study in oral and oropharyngeal cancer patients a strong sense of coherence (a measure of successful coping) was associated with better psychosocial functioning (11). Global QOL did not change after 6 and 12 months, although there was a trend for improvement. Apparently, the negative influence of physical deterioration was counter balanced by the positive changes in emotional functioning, indicating that the sensitivity of the global QOL score may be compromised by a cancellation effect of different QOL domains.
Wang et al. (18) in their study found that the post-operative QOL in patients was significantly influenced by the size of tongue resection. As the resected size of tongue increases, psychological impact increases.
Surgery with RT for cancer of the oral tongue leads to mixed results, in some domains (pain, overall activeness of patient, recreational activities, mood and anxiety) QOL was significantly improved and in some domains like appearance of the patient, swallowing, chewing, speech, shoulder pain, taste and saliva production were worsen. But if we compare the improved and worsen domains we found that improved domains are more important in QOL than the worsen domains.
Conclusions
By our study we can strongly recommend that oral tongue cancers if diagnosed at early stages and without any neck node (T1-2N0M0) can be easily managed without compromising the QOL of the patients significantly.
Acknowledgements
Authors’ contribution: All the authors equally contributed in making the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Scully C, Bedi R. Ethnicity and oral cancer. Lancet Oncol 2000;1:37-42. [PubMed]
- Breitbart W, Holland J. Psychological aspects of head and neck cancer. Semin Oncol 1988;15:61-9. [PubMed]
- Aaronson NK, Bullinger M, Ahmedzai S. A modular approach to quality of life assessment in cancer clinical trials. Recent Results Cancer Res 1988;111:231-49. [PubMed]
- Gamba A, Romano M, Grosso IM, et al. Psychosocial adjustment of patients surgically treated for head and neck cancer. Head Neck 1992;14:218-23. [PubMed]
- Espie CA, Freedlander E, Campsie LM, et al. Psychological distress at follow-up after major surgery for intra-oral cancer. J Psychosom Res 1989;33:441-8. [PubMed]
- Baile WF, Gibertini M, Scott L, et al. Depression and tumor stage in cancer of the head and neck. Psycho-oncology 1992;1:15-24.
- Telfer MR, Shepherd JP. Psychological distress in patients attending an oncology clinic after definitive treatment for maxillofacial malignant neoplasia. Int J Oral Maxillofac Surg 1993;22:347-9. [PubMed]
- Infante-Cossio P, Torres-Carranza E, Cayuela A, et al. Quality of life in patients with oral and oropharyngeal cancer. Int J Oral Maxillofac Surg 2009;38:250-5. [PubMed]
- Weymuller EA, Alsarraf R, Yuch B, et al. Analysis of the performance charcterstics of the University of Washington Quality of life Instrument and its modification (UW-QOL-R). Arch Otolaryngol Head Neck Surg 2001;127:489-93. [PubMed]
- Singer S, Arraras J, Chie WC, et al. Performance of the EORTC questionnaire for the assessment of quality of life in head and neck cancer patients EORTC QLQ-H&N35. A methodological review. Qual Life Res 2013;22:1927-41. [PubMed]
- Langius A, Bjorvell H, Lind MG. Functional status and coping in patients with oral and pharyngeal cancer before and after surgery. Head Neck 1994;16:559-68. [PubMed]
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health related quality of life scores. J Clin Oncol 1998;16:139-44. [PubMed]
- Funk GF, Karnell LH, Dawson CJ, et al. Baseline and post treatment assessment of the general health status of head and neck cancer patients compared with United States population norms. Head Neck 1997;19:675-83. [PubMed]
- Deleyiannis FW, Weymuller EA Jr, Coltrera MD. Quality of life of disease free survivors of advanced (stageIII and IV) oropharyngeal cancer. Head Neck 1997;19:466-73. [PubMed]
- Lazarus CL, Husaini H, Anand SM, et al. Tongue strength as apredictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol 2013;122:386-97. [PubMed]
- López-Jornet P, Camacho-Alonso F, López-Tortosa J, et al. Assessing quality of life in patients with head and neck cancer in Spain by means of EORTC QLQ-C30 and QLQ-H&N35. J Craniomaxillofac Surg 2012;40:614-20. [PubMed]
- Rapoport Y, Kreitler S, Chaitchik S, et al. Psychosocial problems in head and neck cancer patients and their change with time since diagnosis. Ann Oncol 1993;4:69-73. [PubMed]
- Wang J, Luo H, Liu F, et al. Quality of life in oral cancer patients-effects of tongue resection and sociocultural aspects. J Craniofac Surg 2013;24:e493-6. [PubMed]