
Effect of levocetirizine hydrochloride on the growth of human dermal papilla cells: a preliminary study
Introduction
Androgenetic alopecia (AGA) is an androgen-dependent hereditary disorder influenced by a variety of genetic and environmental factors. AGA is the most common type of alopecia and has a chronic disease course, with its main symptoms being progressive hair loss and hair follicle atrophy, which may be accompanied by greasy scalp, dandruff, and itching. Since hair loss directly affects the patient’s appearance, it often has a significant impact on the patient's social activities and mental health and may lead to a decreased quality of life, while worsening social barriers and causing depression in severe cases (1). With recent years of socioeconomic development, many patients have pursued a more positive outcome, and the role of AGA treatment has thus been increasingly recognized.
The etiology and mechanism of AGA are not fully understood. Hair follicle atrophy, miniaturization, shortened anagen phase, prolonged telogen phase, hair follicle involvement, and abnormal growth cycle may be the key links during its pathogenesis. It is well believed that the occurrence and development of AGA are closely related to endocrine disorders, especially the abnormal metabolism of androgen in the hair follicles on the scalp (2). Drugs for AGA include hormone regulators, vasodilators, and immunomodulators; however, few drugs that can effectively promote hair growth have been available. The drugs that are commonly used for AGA are still finasteride and minoxidil; however, finasteride has been reported to cause sexual side effects such as loss of libido and impotence, whereas the use of minoxidil has been associated with irritant dermatitis, allergic contact dermatitis, headache, and hypotension (3). Although many new methods have been developed for AGA treatment including autologous hair transplantation, hair follicle reconstruction, and low-energy laser therapy (4), these are expensive and invasive and thus cannot be widely applied.
Levocetirizine hydrochloride is a safe, highly selective, and highly affinitive new-generation histamine H1 receptor antagonist that has been widely used in the treatment of skin, respiratory, and ocular allergies. It selectively, competitively, and irreversibly binds to transmembrane G protein-coupled receptors and thus inhibits the release of various inflammatory mediators associated with allergy. As the R-enantiomer of cetirizine, levocetirizine hydrochloride has higher bioavailability and lower hepatic clearance, and its plasma binding rate can reach 95%. It is characterized by low tissue affinity, low cardiotoxicity, and low sedation effect (5). A recent study (6) revealed that topical use of cetirizine 1% significantly increased total hair density in the balding area, whereas the vellus hair density showed an evident decrease. Another study demonstrated that levocetirizine hydrochloride, by binding to COX-2, inhibited its ability to promote prostaglandin expression (7).
More evidence has suggested that the prostaglandin pathways (especially the PGDS/PGD pathway) are involved in the pathogenesis of AGA (8). Prostaglandins (PG) are a group of unsaturated fatty acids that are enzymatically metabolized. They are widely distributed in various tissues and body fluids and can mediate multiple physiological functions and inflammatory reactions such as cell proliferation, differentiation, and apoptosis. Cyclooxygenase (COX) induces PGs to be converted from arachidonic acid to unstable prostaglandin H2 and then further processed into various biologically active prostaglandins (e.g., prostacyclin, PGD2, PGE2, PGF2a, and PGI2) or thromboxane A2 (TXA2) by the corresponding prostaglandin synthetases. Comparisons of the effects of different PG subtypes on hair growth have demonstrated that PGE2 and PGF2α can promote hair growth, whereas PGD2 can inhibit hair growth and aggravate hair follicle miniaturization (9). Jeong et al. (10) have found that PGD2 leads to the activation of androgen receptors by regulating the DP2 and AKT/GSK3β signaling pathways on hDPCs. Our current study therefore investigated the effects of levocetirizine hydrochloride on the growth, proliferation, and expression of PG-related signaling factors in human hair dermal papilla cells (DPCs) and explored its effects on hair growth along with the related molecular mechanisms.
Methods
Reagents and equipment
The main reagents used in this study included fetal bovine serum (FBS) and Dulbecco's Modified Eagle Medium (DMEM) (Gibco, USA), levocetirizine hydrochloride powder (Huapond Pharm, Chongqing, China), rabbit anti-human α smooth muscle actin (α-SMA) antibody (PTGlab, USA), Cy3-labeled goat anti-rabbit IgG (IgG-Cy3) (Beyotime, Shanghai, China), and methyl thiazolyl tetrazolium (MTT) (Sigma, USA). The reagent kits included cellular RNA, small RNA, DNA, and protein extraction kit (Magan, USA), reverse transcription, and fluorescence quantitative PCR kits (TAKARA, Japan), and BCA protein quantification kit (Beyotime, Shanghai, China). Other reagents included anti-actin antibody, anti-GAPDH antibody, prostaglandin D2 synthase (PTGDS) (Abcam, USA) as well as anti-pAKT antibody and anti-pGSK3β antibody (CST, USA). Kits for PGD2 enzyme-linked immunosorbent assay (ELISA) (Wuhan Huamei, China) and for PGD2 receptor ELISA (Jingmei Biotechnology, Jiangsu, China) were also used. The equipment used included microplate reader (Thermo, USA), fluorescence quantitative PCR system, (Roche, Switzerland), and gel imaging analysis system (GE, USA).
Study methods
Cell culture
Human dermal papilla cells (hDPCs) (Promocell, Germany) were thawed and resuscitated and then mixed with high-glucose DMEM containing 15% FBS (15% conditioned DMEM). The mixture was cultured in an incubator (37 °C and 5% CO2) for 24 h, and the culture medium was changed every 1–2 days. When the cells were approximately 80% confluent, they were passaged and frozen. The frozen cells were resuscitated and cultured, and hDPCs were digested at the second passage and used in the following assays.
Observation of hDPC growth by using the immunofluorescence technique
hDPCs were seeded in 6-well plates with a density of 1.5×105/well. In each well, 2 mL of 15% DMEM conditioned medium was added. After the cells became adherent, the cells were cultured with 15% DMEM-conditioned medium containing levocetirizine hydrochloride 0 (control), 1, 10, 100, 1,000, or 10 000 ng/mL for 48 h. After the cells were mounted and cultured on slides, they were immersed in a phosphate buffer solution with Tween® 20 (PBST), fixed in 4% paraformaldehyde, blocked with 1% BSA for 30 min, and incubated with rabbit anti-human α-SMA antibody in a wet box at 4 °C overnight. After the cells were immersed in PBST again, the mixture was added with IgG-Cy3 and incubated in a dark at room temperature for 1 h. DAPI was added to the wells before observation under a fluorescence microscope. The whole procedure was repeated three times.
Measurement of cell proliferation rate by MTT assay
hDPCs were seeded in 96-well plates at 1×104/well, and each well was mixed with 200 µL of 15% DMEM conditioned medium. After the cells became adherent, the cells were cultured with 15% DMEM-conditioned medium containing levocetirizine hydrochloride 0 (control), 1, 10, 50, 100, 250, 500, 1,000, 10,000, and 100,000 ng/mL for 48 h. Zero wells were set, and five duplicate wells were set for each concentration. After the cells were washed with PBST twice, serum-free DMEM medium containing MTT was added. After the mixture was incubated at 37 °C for 4 h, 150 µL of dimethyl sulfoxide was added in each well. The mixture was incubated with agitation in dark on a plate shaker for 10 min. Absorbance (A value) was measured spectrophotometrically at 490 nm. The whole procedure was repeated three times, and the means were calculated. The cell proliferation rate was calculated using the following formula: the proliferation rate (%) = (the average value of all A values for experimental wells − the average value of all A values for zero wells)/(the average value of all A values for control wells —the average value of all A values for zero wells)×100%.
Determination of the expressions of the mRNA and protein expressions of relevant genes in hDPCs by using real-time fluorescence quantitative PCR and Western blotting
hDPCs were seeded in 6-well plates at 1.5×105/well, and each well was mixed with 2 mL of 15% DMEM-conditioned medium. After the cells became adherent, the cells were cultured with 15% DMEM-conditioned medium containing levocetirizine hydrochloride 0 (control), 1, 10, 100, 1,000, or 10 000 ng/mL for 48 h. After the supernatant was discarded, the total RNA and total protein were extracted by using cellular RNA, small RNA, DNA, and protein extraction kits.
(I) Determination of mRNA expression levels proceeded as follows. Total RNA was synthesized into a cDNA template using a reverse transcription kit, and the target gene primers were synthesized by Shanghai Sangon Biotech using the following sequences (Table 1). PCR amplification was performed according to the manufacturer’s instructions. The reaction system was 20 µL, and the conditions were as follows: Pre-denaturation at 95 °C for 3 min, followed by 45 cycles of denaturation at 95 °C for 3 s and annealing and extension at 60 °C for 30 s. The amplification curve was analyzed after the reaction was ended. With the β-actin gene as an endogenous reference gene, the relative mRNA expression was calculated using the 2−ΔΔCt method. The experiment was repeated three times. The ΔCT value in the treated group = the average CT value of the treated group − the CT value of the endogenous control; the ΔCT value in the blank control group = the average CT value of the blank control group—the CT value of the endogenous control; and the ΔΔCt = the ΔCT value in the treated group—the ΔCT value in the blank control group;

Full table
(II) Western blotting for determining the protein expressions of PTGDS, pAKT, and pGSK3β proceeded as follows. After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the extracted total protein was transferred onto a PVDF membrane. After blocking with 1% bovine serum albumin (BSA) for 1.5 h, the proteins were mixed with the corresponding antibodies (1:1,000) and then inoculated overnight on a plate shaker in a cold room (4 °C); subsequently, the secondary antibodies were added before inoculation at room temperature for 1 h. A gel imaging system was used for imaging. The whole procedure was repeated three times.
ELISA for measuring the PGD2 and PGD2R levels in the supernatant of the cultured hDPCs
hDPCs were seeded in 6-well plates at 2×105/well, and each well had 2 mL of 15% DMEM-conditioned medium added. After the cells became adherent, they were cultured with serum-free DMEM containing levocetirizine hydrochloride 0 (control), 1, 10, 100, 1,000, or 10,000 ng/mL for 24 h. The culture media were collected and centrifuged at 1,000 rpm (radius arm: 15.0 cm) for 5 min. The supernatant was harvested, and the levels of PGD2 and PGD2R were determined according to the kit instructions. The whole procedure was repeated three times.
Statistical analysis
The statistical analysis was performed using the SPSS 17.0 software package. Measurement data are presented as . Comparisons among multiple groups were performed using one-way analysis of variance (ANOVA), and the LSD-t test was used for pairwise comparisons. A P value of <0.05 was considered statistically significant.
Results
hDPC growth
The cells in the 100 ng/mL group grew well and arranged closely, with abundant cytoplasm and over 90% confluency. Cell confluency was about 70–80% in the blank control group. The cells in the 10,000 ng/mL group were sparsely distributed and loosely arranged, with a confluency of less than 60% (Figure 1).
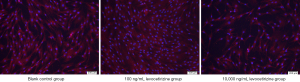
Effect of levocetirizine hydrochloride on the proliferation of hDPCs
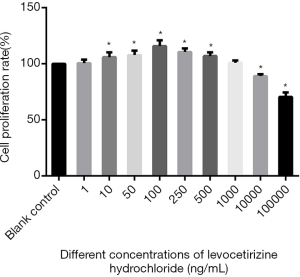
The MTT results showed that the proliferation rate of hDPCs varied significantly across the different groups (F=42.22, P<0.05), It was significantly higher in the 10, 50, 100, 250, 500 ng/mL groups than in the blank control group (the t values were 5.31, 12.68, 28.26, 32.43, and 13.01, respectively; all P<0.05), whereas the 1 and 1,000 ng/mL groups showed no such significant difference when compared with the blank control group (t=0.11, t=0.66, respectively; both P>0.05). The proliferation rate of hDPCs in the 10,000 and 100,000 ng/mL groups was significantly lower than that in the blank control group (t=143.95, t=161.26, respectively; both P<0.05) (Figure 2).
Effects of levocetirizine hydrochloride on the mRNA expressions of COX-2, PGE2, PGF2α, PTGDS, GPR44, AKT, and GSK3β
The mRNA expressions of COX-2, PGF22, PTGDS, GPR44, and AKT showed significant differences in different levocetirizine hydrochloride groups (all P<0.05); the mRNA expressions of COX-2, PTGDs, and GPR44 mRNA in the 100 ng/mL group (0.84±0.08, 0.81±0.10, and 0.85±0.09, respectively) were significantly lower than those in the blank control group (t=1.97, t=2.17, and t=2.66, respectively; all P<0.05), whereas the mRNA expressions of PGF2α and AKT in the 100 ng/mL group (1.96±0.25 and 1.74±0.32, respectively) were significantly higher than those in the blank control group (t=3.66 and t=7.33, respectively; both P<0.05). However, the mRNA expressions of PGE2 and GSK3β showed no significant difference between the different levocetirizine hydrochloride groups (all P>0.05) (Table 2).

Full table
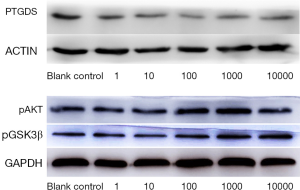
Effects of levocetirizine hydrochloride on the protein expressions of PTGDS, pAKT, and pGSK3β in hDPCs
There were significant differences in the levels of PTGDS, pAKT, and pGSK3β proteins among different levocetirizine hydrochloride groups (the F values were 11.84, 3.89, and 4.07, respectively; P<0.05). The level of PTGDS protein in each concentration group was significantly lower than that in the blank control group (the t values were 11.58, 12.54, 5.67, 8.42, and 8.16, respectively; P<0.05). The levels of pAKT protein in the 100 and 1,000 ng/mL groups were significantly higher than those in the blank control group (t=16.60, t=12.42, respectively); however, the pAKT protein levels in the 1, 10, and 10,000 ng/mL groups were not significantly different from those in the blank control group (t=0.28, t=0.03, and t=0.22, respectively; all P>0.05). The levels of pGSK3β protein in the 100, 1,000, and 10,000 ng/mL groups were significantly higher than those in the blank control group (t=7.73, t=8.91, and t=5.94, respectively); however, the pGSK3β protein levels in the 1 and 10 ng/mL groups were not significantly different from those in the blank control group (t=0.004, t=0.03, respectively; both P>0.05) (Figure 3 and Table 3).
Full table
Effect of levocetirizine hydrochloride on the secretion of PGD2 and PGD2 receptors in hGDC
As shown by ELISA, the levels of PGD2 and PGD2R receptors in hDPC culture supernatants were significantly different across the different levocetirizine hydrochloride groups (all P<0.05). The levels of PGD2 and PGD2R proteins in the 10–10,000 ng/mL groups were significantly lower than those in the blank control group (the t values were 20.75, 45.07, 87.09, 101.62, 46.72, 92.05, 90.67, and 83.16, respectively; all P<0.05), although they were not significantly different between the 1 ng/mL group and the blank control group (t=0.26, t=1.72, respectively; both P>0.05) (Table 4).
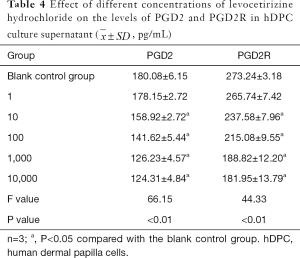
Full table
Discussion
DPCs, located in the dermal papilla at the base of the hair follicle, are a group of specially differentiated mesenchymal cells. Studies have confirmed that DPCs can induce the proliferation and differentiation of epithelial cells to form hair follicles, regulate hair growth cycle, and thus play an important role in the pathogenesis of AGA. It is now widely accepted that hair follicle stem cells are activated after receiving signals from the dermal papilla, which activates the hair follicles and triggers the hair to shift from the stationary phase into the growth phase (11). Abnormal androgen metabolic pathway is the primary triggering factor of AGA. Androgen receptors are mainly located in DPCs, further indicating that DPCs are the main target cells of androgen action on hair follicles (2). In vitro culture has shown that DPCs induce the proliferation and differentiation of epithelial cells through Wnt/β-catenin signaling pathway and promote the formation of hair follicles (12). In addition, DPCs can promote and maintain the growth and development of hair follicles by secreting various cytokines and growth factors; particularly, the signal molecules including FGF, TGF-β1, IGF-1, VEGF, BMP, and DKK-1, which are produced by autocrine or paracrine, can interweave to form complex regulatory networks (13). Therefore, many studies have tended to use DPCs as an in vitro screening model to explore the mechanisms of hair growth regulators at the cellular and molecular levels.
No experiment has explored the effect of levocetirizine hydrochloride on hDPCs. In 2015, Wichitnithad et al. (14) found that, after healthy subjects were orally administered with levocetirizine hydrochloride at a therapeutic dose of 5 mg, the Cmax fluctuated at 196±36 ng/mL, as determined by fast liquid chromatography-mass spectrometry. In the study performed by Jang et al. (15), the peak concentration of cetirizine in the epidermis of the skin reached 1.6–2.4 ng/mL after oral administration of 10 mg or 20 mg of cetirizine in healthy subjects. Petersen et al. (16) infected human nasal mucosal epithelial cells with human rhinovirus (HPV) to mimic a model of airway inflammation, which was treated with different concentrations of levocetirizine hydrochloride (molecular weight 388 g/mol); it was found that 19.4 ng/mL levocetirizine hydrochloride could effectively inhibit the expression of inflammatory cytokines including IL-6, IL-8, and NF-κβ induced by HPV. We selected drug dilution concentrations using the above intervals, with the highest concentration being 0.01% levocetirizine hydrochloride (100,000 ng/mL). Both α-SMA immunofluorescence staining and MTT assay showed that levocetirizine hydrochloride significantly promoted the proliferation of hDPCs, with good cell growth and a significantly increased proliferation rate at the concentration of 100 ng/mL. These findings suggest that levocetirizine hydrochloride might promote the growth and proliferation of hDPCs through the regulation of certain cell secreting factors, signaling molecules, and signal transduction pathways.
Many recent articles have described the role of prostaglandins in hair growth and development. Garza et al. (17) confirmed that the mRNA and protein expression levels of both PTGDS and PGD2 increased in the alopecia area of AGA patients, particularly when the hair at the hair follicle was in a transition period between growth and regression. They also demonstrated that the tropical use of PGD2 in mice transplanted with human hair follicles led to the miniaturization of hair follicles by inhibiting GPR44, resulting in hair thinning and shedding (18). PGD2 is an important inflammatory regulatory molecule that enhances or inhibits inflammatory responses by interacting with D prostanoid receptor 1 (PTGDR), D prostanoid receptor 2 (PGD2R), and TP receptors. In general, as a pro-inflammatory factor, PGD2 activates the DP2 receptor in human airway epithelial cells, stimulates massive mucus secretion by airway epithelial cells, causes bronchial muscle contraction, and leads to airway hyperresponsiveness (19). Recently, some studies have suggested that PGD2 may inhibit inflammatory response in certain environments. For instance, it can activate DP1 receptor to regulate and induce lung dendritic cells and regulatory T cells, and ultimately reduce asthma attacks. As a receptor for PGD2, GPR44 is expressed not only in the outer root sheath of the hair follicle but also in the dermal papilla of the outer sheath region (20-21). In addition, overexpression of COX-2 in mouse models also causes hair loss (22). Treatment of AGA using PTGDS inhibitors has been reported (23). These results suggest that prostaglandins are involved in hair growth and may be a new therapeutic target for alopecia.
In 1989, Charlesworth et al. found that cetirizine could inhibit inflammatory cell infiltration and while also significantly reducing the expression of PGD2 (24). Levocetirizine hydrochloride, by binding to COX-2, inhibits its ability to promote prostaglandin expression (7). Such effects are clearly independent of their antihistaminic activity. Recently, Jeong et al. (25) found that PGD2 activates androgen receptors by regulating DP2 and AKT signaling pathways on hDPCs, thereby stimulating the production of hair growth inhibitory factors such as TGFβ1, Creb, LEF1, and IGF-1. AKT, also known as protein kinase B, is an important signal transduction molecule that regulates cell proliferation, differentiation, migration, and apoptosis. For one, the AKT signaling pathway promotes the proliferation of hair follicle stem cells and induces epidermal hyperplasia (26). For another, the activation of AKT signaling pathway activates multiple downstream target protein genes including GSK3β, Bcl-2 family members, caspase-9, and NF-kB, and thus participates in the proliferation and differentiation of DPCs and the growth of hair follicles. GSK3β, for example, is both a downstream gene in the AKT signaling pathway and also a key factor in the classical Wnt/β-catenin signaling pathway (which is the first dermal signaling pathway).
In our current study, we used hDPCs to explore the relationship between levocetirizine hydrochloride and hair growth. Real-time quantitative PCR showed that levocetirizine hydrochloride 100 ng/mL significantly inhibited the mRNA expressions of COX-2, PTGDS, and GPR44 and promoted the mRNA expression of AKT. Western blotting and ELASA also showed that levocetirizine hydrochloride reduced the protein expression levels of PTGDS, PGD2, and PGD2 receptors while up-regulating the protein expression levels of pAKT protein and pGSK3β. In addition, comparisons of the effects of different concentrations of levocetirizine hydrochloride on hDPCs showed that low- and high-concentrations of levocetirizine hydrochloride were not conducive to the expressions of the above signaling molecules and pathway molecules. For example, levocetirizine hydrochloride 1 ng/mL could only down-regulate the protein expression of PTGDS in hDPCs, whereas levocetirizine hydrochloride 10,000 ng/mL significantly inhibited the protein expressions of PTGDS, PGD2, and PGD2R. Therefore, levocetirizine hydrochloride may promote the growth and proliferation of hDPC in vitro by inhibiting the PGD2-GPR44 pathway and activating the AKT signaling pathway.
In addition, we found that levocetirizine hydrochloride promoted the expression of PGF2α but did not affect the expression of PGE2. Although both of them are prostaglandins, their effects on hair growth are exactly the opposite of those of PGD2. In 2009, the US Food and Drug Administration (FDA) approved the clinical use of Latanoprost, a PGF2α analogue, to promote the growth of human eyelashes and eyebrows (27). It is believed that PGF2α and its analogues promote the proliferation of hair follicle epithelial cells mainly by relaxing the small arteries and thus increasing local blood supply (28). PGE2 has also been shown to improve hair loss induced by radiation in mouse models (29). At present, no experiment has investigated the downstream signaling pathway of AKT and the regulation mechanism of PGF2α. Moreover, the mechanism via which levocetirizine hydrochloride regulates PDC2-GPR44 in living bodies also warrants further study.
In conclusion, our experiment demonstrated that levocetirizine hydrochloride could promote the growth and proliferation of hDPCs and significantly inhibit the expressions of relevant factors including COX-2, PTGDS, PGD2, PGD2R, and GPR44 in the PGD2-GPR44 pathway. The role of AKT signaling pathway in this process was also explored. These findings provide an experimental and theoretical basis for the treatment of AGA with levocetirizine hydrochloride.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81502744) and the Guangxi Natural Science Foundation (2015GXNSFBA139131).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamazaki M, Miyakura T, Uchiyama M, et al. Oral finasteride improved the quality of life of androgenetic alopecia patients. J Dermatol 2011;38:773-7. [Crossref] [PubMed]
- Urysiak-Czubatka I, Kmieć ML, Broniarczyk-Dyła G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postepy Dermatol Alergol 2014;31:207-15. [Crossref] [PubMed]
- Zhang Y. A study on the difference between normal hair follicle and pathological hair follicle in males with alopecia. Second Military Medical University, 2016.
- Ghanaat M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med J 2010;103:917-21. [Crossref] [PubMed]
- Kanei A, Asano K, Kanai K, et al. Inhibitory action of levocetirizine on the production of eosinophil chemoattractants RANTES and eotaxin in vitro and in vivo. In Vivo 2014;28:657-66. [PubMed]
- Rossi A, Campo D, Fortuna MC, et al. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. J Dermatolog Treat 2018;29:149-151. [Crossref] [PubMed]
- Abo Dena AS, Abdel Gaber SA. In vitro drug interaction of levocetirizine and diclofenac: Theoretical and spectroscopic studies. Spectrochim Acta A Mol Biomol Spectrosc 2017;181:239-48. [Crossref] [PubMed]
- Arias-Santiago S, Arrabal-Polo MA, Buendía-Eisman A, et al. Androgenetic alopecia as an early marker of benign prostatic hyperplasia. J Am Acad Dermatol 2012;66:401-8. [Crossref] [PubMed]
- Heilmann S, Nyholt DR, Brockschmidt FF, et al. No genetic support for a contribution of prostaglandins to the aetiology of androgenetic alopecia. Br J Dermatol 2013;169:222-4. [Crossref] [PubMed]
- Jeong KH, Jung JH, Kim JE, et al. Prostaglandin D2-Mediated DP2 and AKT Signal Regulate the Activation of Androgen Receptors in Human Dermal Papilla Cells. Int J Mol Sci 2018. [Crossref] [PubMed]
- Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009;4:155-69. [Crossref] [PubMed]
- Kwack MH, Kim MK, Kim JC, et al. Wnt5a attenuates Wnt/β-catenin signalling in human dermal papilla cells. Exp Dermatol 2013;22:229-31. [Crossref] [PubMed]
- Madaan A, Verma R, Singh AT, et al. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int J Cosmet Sci 2018;40:429-50. [Crossref] [PubMed]
- Wichitnithad W, Jithavech P, Sanphanya K, et al. Determination of levocetirizine in human plasma by LC-MS-MS: validation and application in a pharmacokinetic study. J Chromatogr Sci 2015;53:1663-72. [Crossref] [PubMed]
- Jang YJ, Wang JH, Kim JS, et al. Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells. Antiviral Res 2009;81:226-33. [Crossref] [PubMed]
- Petersen LJ, Church MK, Rihoux JP, et al. Measurement of interstitial cetirizine concentrations in human skin: correlation of drug levels with inhibition of histamine-induced skin responses. Allergy 1999;54:607-11. [Crossref] [PubMed]
- Garza LA, Liu Y, Yang Z, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med 2012;4:126ra34. [Crossref] [PubMed]
- Nieves A, Garza LA. Does prostaglandin D2 hold the cure to male pattern baldness?. Exp Dermatol 2014;23:224-7. [Crossref] [PubMed]
- Chen LX, Ran DH, Huang HP, et al. Prostaglandin D2 stimulates human airway mucus hypersecretion mediated by DP2. Basic & Clinical Medicine 2016;36:24-9.
- Colombe L, Vindrios A, Michelet JF, et al. Prostaglandin metabolism in human hair follicle. Exp Dermatol 2007;16:762-9. [Crossref] [PubMed]
- Colombe L, Michelet JF, Bernard BA. Prostanoid receptors in anagen human hair follicles. Exp Dermatol 2008;17:63-72. [PubMed]
- Bol DK, Rowley RB, Ho CP, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res 2002;62:2516-21. [PubMed]
- Fong P, Tong HH, Ng KH, et al. In silico prediction of prostaglandin D2 synthase inhibitors from herbal constituents for the treatment of hair loss. J Ethnopharmacol 2015;175:470-80. [Crossref] [PubMed]
- Charlesworth EN, Kagey-Sobotka A, Norman PS, et al. Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reaction. J Allergy Clin Immunol 1989;83:905-12. [Crossref] [PubMed]
- Jeong KH, Jung JH, Kim JE, et al. Prostaglandin D2-Mediated DP2 and AKT Signal Regulate the Activation of Androgen Receptors in Human Dermal Papilla Cells. Int J Mol Sci 2018. [Crossref] [PubMed]
- Ohnemus U, Uenalan M, Inzunza J, et al. The hair follicle as an estrogen target and source. Endocr Rev 2006;27:677-706. [Crossref] [PubMed]
- Faghihi G, Andalib F, Asilian A. The efficacy of latanoprost in the treatment of alopecia areata of eyelashes and eyebrows. Eur J Dermatol 2009;19:586-7. [PubMed]
- Barrón-Hernández YL, Tosti A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs 2017;26:515-22. [Crossref] [PubMed]
- Geng L, Hanson WR, Malkinson FD. Topical or systemic 16, 16 dm prostaglandin E2 or WR-2721 (WR-1065) protects mice from alopecia after fractionated irradiation. Int J Radiat Biol 1992;61:533-7. [Crossref] [PubMed]


