
Effect of bipolar pulsed radiofrequency on chronic cervical radicular pain refractory to monopolar pulsed radiofrequency
Introduction
Cervical radicular pain due to spinal disease is one of most common forms of neuropathic pain, affecting approximately 0.8 in 1,000 people per year (1). The mechanical compression of the sensory nerve roots and the resulting inflammation, caused by herniated cervical disc (HCD) and cervical foraminal stenosis, are the main causes of cervical radicular pain (2-4). For the management of cervical radicular pain resulting from spinal disease, various oral medications and treatment modalities are currently applied in clinical practice. Of these, transforaminal epidural steroid injections (TFESI) are one of most effective methods for managing cervical radicular pain (2,5). Steroids inhibit the synthesis of various proinflammatory mediators and reduce nerve root inflammation caused by mechanical compression at the narrowed cervical foramen (2,4,6). However, despite the application of these conservative treatments, many patients complain of persisting cervical radicular pain symptoms. The treatment outcomes of persistent chronic cervical radicular pain have, to date, not been favorable for patients.
Pulsed radiofrequency (PRF), a relatively recently introduced therapeutic procedure, is reported to safely and effectively control various types of chronic neuropathic pain (7-9). PRF uses radiofrequency currents to produce heat bursts, which are subsequently delivered to targeted nerves without causing significant damage to neural structures (10-12). In contrast, continuous radiofrequency (CRF) exposes target nerves to a continuous electrical stimulation, which subsequently increases the temperature around the radiofrequency needle tip and ablates the surrounding neural structures (13). PRF applies a brief electrical stimulation that is followed by a long resting phase. Therefore, during PRF stimulation, although heat is produced, the heat intensity is not sufficient enough to cause any significant structural damage (14). The proposed mechanism of PRF is that the electrical field produced by PRF hinders the transmission of noxious signals and alters pain perception (15-17). To date, several previous studies have reported that PRF stimulation of the dorsal root ganglion (DRG) can successfully control cervical radicular pain that results from spinal disease (18-24). However, in clinical practice, despite the beneficial effects of PRF, some patients’ radicular pain continues to be insufficiently managed. Conventionally, for controlling neuropathic pain, a single cannula (monopolar PRF) is used. However, to overcome the therapeutic limitations of the monopolar PRF, some previous studies applied two electrode tips (bipolar PRF) in refractory neuropathic pain conditions because bipolar PRF produces denser and larger electrical fields (9,18,25). However, to date, little is known about the effects of bipolar PRF treatment in cervical radicular pain.
In the current study, we applied bipolar PRF stimulation on cervical DRG in patients with chronic cervical radicular pain resulting from spinal disease who were unresponsive to both monopolar PRF of DRG and TFESI, and evaluated its effectiveness for 3 months following bipolar PRF stimulation.
Methods
Patients
From September 2017 to January 2019, a total of 60 patients with chronic cervical radicular pain received monopolar PRF stimulation of their cervical DRG, and we prospectively recruited patients who continued to present with persistent cervical radicular pain after a monopolar PRF procedure applied using C-arm fluoroscopy. Monopolar PRF was performed when the patient’s radicular pain was scored at least 4 (0 indicating no pain and 10 indicating the worst pain imaginable) on a Numeric Rating Scale (NRS), despite having been exposed to ≥1 cervical TFESI procedure. Twenty-four patients of 60 patients had persistent cervical radicular pain of at least NRS 4. Of these 24 patients, 20 patients (mean age: 60.8±7.9 years, range 44–72 years) were included in this study after applying inclusion and exclusion criteria and received bipolar PRF stimulation on their DRG (Table 1). Inclusion criteria were as follows: (I) presentation with ≥6-month history of segmental pain of cervical origin radiating from the neck to the arm or hand; (II) age 20–79 years; (III) ≥80% temporary pain relief following a diagnostic nerve block with 1 mL of 2% lidocaine; (IV) unsatisfactory response to monopolar PRF stimulation of the DRG (pain of ≥4 on NRS); (V) no interval change in the pain score on the NRS over the 4 weeks following monopolar PRF; (VI) imaging findings (magnetic resonance imaging and/or computed tomography) of HCD or cervical foraminal stenosis compatible with the patient’s pain symptoms. We excluded patients through following criteria: (I) previous history of cervical spinal surgery; (II) presence of peripheral neuropathy other than cervical radiculopathy, cervical myelopathy, or infection on the spine; (III) bilateral symptoms or involvement of >1 segment, and (IV) the presence of a coagulation disorder. The Institutional Review Board of our hospital approved the study, and all patients provided a signed informed consent form.
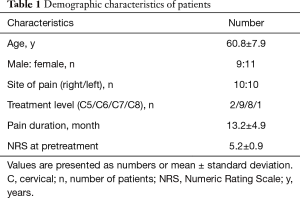
Full table
Bipolar PRF procedures
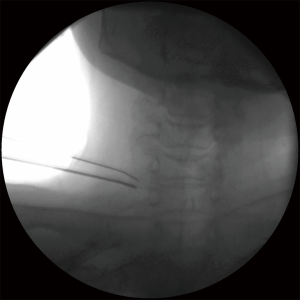
The bipolar PRF procedure was performed under aseptic conditions. The patient was laid in a supine position for C-arm fluoroscopy (Siemens, Munich, Germany). Two 22-gauge curved-tip catheters (SMK pole needle, 100 mm with a 10 mm active tip, Cotop International BV) were inserted and a sensory stimulation test was conducted using a radiofrequency generator (Cosman G4, Cosman Medical, Burlington, MA, USA). Each catheter needle was then advanced toward the DRG until the patient reported a tingling sensation and/or dysesthesia at <0.3 V. The distance between the 2 catheter needle tips was <10 mm (26), but they were not in contact with each other (Figure 1). The PRF treatment was administered at 5 Hz and a 5-ms pulsed width for 360 seconds at 45 V, with the constraint that the electrode tip temperature did not exceed 42 °C. The physician, who had 10-years’ experience of spinal interventions, conducted all the bipolar PRF procedures and was blinded to the outcome measurements.
Outcome measures
One investigator performed all the pretreatment and follow-up assessments and did not participate in conducting any treatment procedures. Pain intensities were assessed using an NRS (ranged from 0–10).
NRS scores were assessed before treatment and at 1, 2, and 3 months following treatment. Successful treatment was defined as a ≥50% reduction in the baseline NRS score at 3 months. The percentage of reduction in NRS scores was quantified by expressing score reductions at 3 months as percentages of baseline scores. Additionally, patient global perceived effect (GPE) was assessed at 3 months post-PRF using a 7-point Likert scale (27,28) (Table 2), and patients that reported very good (score =7) or good results (score =6) were considered to be satisfied with the procedure.

Full table
Statistical analysis
SPSS Version 23.0 (IBM Corporation, Armonk, NY, USA) was used for data analysis. The summary of characteristic variables was performed using descriptive analysis, with the mean ± standard deviation presented for quantitative variables and frequency (percent) for qualitative variables. Changes in NRS scores over time were evaluated using repeated measures one-factor analysis. Multiple comparison results were obtained following a contrast under Bonferroni correction. The level of statistical significance was set at P values <0.05.
Results
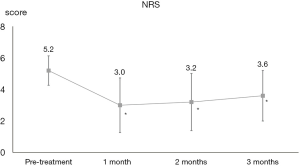
All included patients completed the study protocol and did not present with any adverse events. The average NRS score for cervical radicular pain declined from 5.2±0.9 at baseline to 3.0±1.7 at 1 month, 3.2±1.8 at 2 months, and 3.6±1.6 at 3 months after the PRF procedure. NRS scores changed significantly over time (P<0.001) (Figure 2). More specifically, NRS scores at 1, 2, and 3 months after PRF were significantly lower than at baseline (P<0.001) (Figure 2). Ten of the 20 patients (50%) reported successful pain relief (≥50%) at 3 months after PRF.
Patient’s satisfaction with treatment, as evaluated using the 7-point Likert scale, was as follows: very good (score =1) in 1 patient (5%), good (score =6) in 9 patients (45%), and fairly good (score =5) in 2 patients (10%). No change (score =4) was reported by 8 patients (40%). No patient reported experiencing fairly bad (score =3), bad (score =2), or very bad (score =1) treatment satisfaction.
Therefore, half of all included patients (10 out of 20 patients) were satisfied with bipolar PRF of cervical DRG at 3 months following the procedure.
Discussion
In this study, we evaluated the effect of bipolar PRF stimulation of the DRG in people with chronic cervical radicular pain who were unresponsive to both monopolar PRF of DRG and cervical TFESI. Pain severity scores were significantly reduced at 1, 2, and 3 months following bipolar PRF. Half of the patients demonstrated a successful response (≥50% pain reduction) and were satisfied with their treatment results at 3 months after the procedure.
The mechanism of action of PRF on alleviating pain has not yet been clearly established, but some mechanisms have been proposed. Cosman et al. suggested that the low frequency of pulses and the high voltages in PRF induces long-term depression of synaptic transmission, subsequently preventing the transfer of noxious signals to the brain (29). Erdine et al. reported that PRF leads to microscopic damage (abnormal membranes and morphology of mitochondria, and disruption and disorganization of microfilaments and microtubules) in the principal sensory nociceptive sensory fibers (C-fibers and A-delta fibers), but rarely causes damage to the larger non-pain-related sensory fibers (A-beta fiber) (30). Higuchi et al. found the application of PRF to DRG increases c-fos in the dorsal horn, which is known to sustain activation of some pain-inhibitory mechanisms (15). Cho et al. reported that PRF stimulation of the DRG decreases microglia activity in the dorsal horn (16). Activation of microglia plays an important role in the development of chronic neuropathic pain by secreting several inflammatory cytokines and chemokines that mediate pain signaling, thus causing a downregulation of microglia activity and could hinder the progression to chronic neuropathic pain.
Recently, based on above suggested evidence, monopolar PRF is widely used to alleviate various types of neuropathic pain in clinical practice. However, it has been suggested that bipolar PRF would be more effective than monopolar PRF because bipolar PRF would produce denser and larger electrical fields. Cosman et al. showed that the parallel-tip used during bipolar RF produced larger-sized lesions compared with monopolar RF (31). The 10 mm uninsulated catheter tip using during monopolar CRF stimulation produces a small prolate spheroid lesion of 12.8 mm × 7.8 mm (length × width) around its tip (31). In contrast, bipolar CRF generates a lesion between and around 2 closely positioned catheter tips. When 2 parallel catheter tips were 10 mm apart from each uninsulated catheter tips during bipolar CRF, the lesion size was 15.5 mm × 11.8 mm (length × width) (31). Therefore, bipolar RF can cover the targeted area more sufficiently. Direct comparison between CRF and PRF may be difficult, but the results of CRF could be similarly applied to PRF. Based on this idea, we performed bipolar PRF on DRG of patients with chronic cervical radicular pain who were unresponsive to monopolar PRF. Our patients’ radicular pain was significantly reduced, and this effect was sustained for ≥3 months following treatment. Also, half of included patients were satisfied with the results of bipolar PRF treatment at 3 months after the procedure.
Considering that our patients’ average duration from pain onset to bipolar PRF procedure was 13.2 months and all the patients’ pain duration was >6 months, the pain levels of our patients appeared to have reached a plateau state. Therefore, the reduction in pain is not likely to be responsible for the natural process of cervical radicular pain. Accordingly, although we did not compare our results with a control group, we believe that the pain reductions in our patients were attributed to application of the bipolar PRF.
To the best of our knowledge, for the management of chronic neuropathic pain, so far, 3 previous studies conducted bipolar PRF (9,18,25). In 2017, Chang et al. recruited 50 patients with chronic lumbosacral radicular pain who were randomly assigned to either the bipolar PRF or monopolar PRF group (25). They reported that the patients who received bipolar PRF demonstrated superior pain relief compared with monopolar PRF during the 3-month follow-up period. In the same year, Chang et al. performed bipolar PRF on cervical DRG to 2 patients with chronic cervical radicular pain who were unresponsive to monopolar PRF and repeated TFESIs (18). At 6 months after the procedure, pre-treatment pain degrees of NRS 7 and 6 in each patient were reduced to NRS 2. In 2018, Lee et al. conduced bipolar PRF on lumbar DRG in 23 patients with chronic lumbosacral radiculopathy who were unresponsive to monopolar PRF and TFESI (9). After the bipolar PRF, the pre-treatment NRS was significantly reduced, and this effect persisted for ≥3 months after the procedure. At 3 months, 52.2% of the 23 patients demonstrated a >50% pain reduction and were satisfied with their treatment results. In all of these previous studies using bipolar PRF, no major adverse effects were observed in any patient, similar to our study findings. Therefore, our study is the first prospective clinical trial to evaluate the effect of bipolar PRF on DRG in patients with refractory cervical radicular pain.
In conclusion, we found that cervical radicular pain refractory to monopolar PRF and TFESI was significantly reduced at 1, 2, and 3 months after bipolar PRF on DRG. Furthermore, half of our patients demonstrated a clinically relevant reduction in pain levels and were satisfied with bipolar PRF at 3 months following the procedure. In clinical practice, if monopolar PRF or TFESI fail to control cervical radicular pain, physicians have limited options to control this pain conservatively, and should, therefore, consider surgical operation as the next treatment option. Therefore, we believe that bipolar PRF of DRG could be considered a safe modality for alleviating refractory chronic cervical radicular pain. However, our study has some limitations. First, as mentioned above, this study was performed without a control group. Second, the number of recruited patients was relatively small. Third, the long-term effects of bipolar PRF were not evaluated. Lastly, we could not clearly explain why bipolar PRF demonstrated a better treatment outcome than monopolar PRF. In the future, further studies that address these limitations are warranted.
Acknowledgments
Funding: The present study was supported by a National Research Foundation of Korea grant funded by the Korean government (grant No. NRF-2019R1F1A1061348).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Yeungnam University (No. 2017-02-011), and all patients provided a signed informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Radhakrishnan K, Litchy WJ, O'Fallon WM, et al. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain 1994;117:325-35. [Crossref] [PubMed]
- Kim MS, Lee DG, Chang MC. Outcome of Transforaminal Epidural Steroid Injection According to Severity of Cervical Foraminal Stenosis. World Neurosurg 2018;110:e398-403. [Crossref] [PubMed]
- Nahm FS, Lee PB, Choe GY, et al. Therapeutic effect of epidural hyaluronic acid in a rat model of foraminal stenosis. J Pain Res 2017;10:241-8. [Crossref] [PubMed]
- Olmarker K, Byrod G, Cornefjord M, et al. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine 1994;19:1803-8. [Crossref] [PubMed]
- Thomas E, Cyteval C, Abiad L, et al. Efficacy of transforaminal versus interspinous corticosteroid injectionin discal radiculalgia—a prospective, randomised, double-blind study. Clin Rheumatol 2003;22:299-304. [Crossref] [PubMed]
- Chang MC, Lee DG. Outcome of Transforaminal Epidural Steroid Injection According to the Severity of Lumbar Foraminal Spinal Stenosis. Pain Physician 2018;21:67-72. [Crossref] [PubMed]
- Chang MC. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain Physician 2018;21:E225-34. [Crossref] [PubMed]
- Lee DG, Chang MC. The Effect of Caudal Epidural Pulsed Radiofrequency Stimulation in Patients with Refractory Chronic Idiopathic Axonal Polyneuropathy. Pain Physician 2018;21:E57-62. [PubMed]
- Lee DG, Cho YW, Ahn SH, et al. The Effect of Bipolar Pulsed Radiofrequency Treatment on Chronic Lumbosacral Radicular Pain Refractory to Monopolar Pulsed Radiofrequency Treatment. Pain Physician 2018;21:E97-103. [PubMed]
- Podhajsky RJ, Sekiguchi Y, Kikuchi S, et al. The histologic effects of pulsed and continuous radiofrequency lesions at 42°C to rat dorsal root ganglion and sciatic nerve. Spine (Phila Pa 1976) 2005;30:1008-13. [Crossref] [PubMed]
- Vallejo R, Benyamin RM, Kramer J, et al. Pulsed radiofrequency for the treatment of sacroiliac joint syndrome. Pain Med 2006;7:429-34. [Crossref] [PubMed]
- West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: A case series. Pain Practice 2010;10:485-91. [Crossref] [PubMed]
- Vatansever D, Tekin I, Tuglu I, et al. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain 2008;24:717-24. [Crossref] [PubMed]
- Sluijter ME, Cosman ER, Rittmann WB 3rd, et al. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion—a preliminary report. Pain Clin 1998;11:109-17.
- Higuchi Y, Nashold BS Jr, Sluijter M. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery 2002;50:850-5; discussion 856. [Crossref] [PubMed]
- Cho HK, Cho YW, Kim EH, et al. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: Laboratory investigation. J Neurosurg Spine 2013;19:256-63. [Crossref] [PubMed]
- Hagiwara S, Iwasaka H, Takeshima N, et al. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: Roles of descending adrenergic and serotonergic systems. Eur J Pain 2009;13:249-52. [Crossref] [PubMed]
- Chang MC. Effect of bipolar pulsed radiofrequency on refractory chronic cervical radicular pain: A report of two cases. Medicine (Baltimore) 2017;96:e6604. [Crossref] [PubMed]
- Choi GS, Ahn SH, Cho YW, et al. Long-term effect of pulsed radiofrequency on chronic cervical radicular pain refractory to repeated transforaminal epidural steroid injections. Pain Med 2012;13:368-75. [Crossref] [PubMed]
- Choi GS, Ahn SH, Cho YW, et al. Short-term effects of pulsed radiofrequency on chronic refractory cervical radicular pain. Ann Rehabil Med 2011;35:826-32. [Crossref] [PubMed]
- Kwak SG, Lee DG, Chang MC. Effectiveness of pulsed radiofrequency treatment on cervical radicular pain: A meta-analysis. Medicine (Baltimore) 2018;97:e11761. [Crossref] [PubMed]
- Lee DG, Ahn SH, Lee J. Comparative Effectivenesses of Pulsed Radiofrequency and Transforaminal Steroid Injection for Radicular Pain due to Disc Herniation: a Prospective Randomized Trial. J Korean Med Sci 2016;31:1324-30. [Crossref] [PubMed]
- Wang F, Zhou Q, Xiao L, et al. A Randomized Comparative Study of Pulsed Radiofrequency Treatment With or Without Selective Nerve Root Block for Chronic Cervical Radicular Pain. Pain Pract 2017;17:589-95. [Crossref] [PubMed]
- Yoon YM, Han SR, Lee SJ, et al. The efficacy of pulsed radiofrequency treatment of cervical radicular pain patients. Korean J Spine 2014;11:109-12. [Crossref] [PubMed]
- Chang MC, Cho YW, Ahn SH. Comparison between bipolar pulsed radiofrequency and monopolar pulsed radiofrequency in chronic lumbosacral radicular pain: A randomized controlled trial. Medicine (Baltimore) 2017;96:e6236. [Crossref] [PubMed]
- Gauci CA. Manual of RF Techniques. 3rd ed. Cosman Medical, Ridderkerk, 2011:24-5.
- Likert R. A technique for the measurement of attitudes. Arch Psychol 1932;140:5-55.
- Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical scale. Pain 2001;94:149-58. [Crossref] [PubMed]
- Cosman ER Jr, Cosman ER Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med 2005;6:405-24. [Crossref] [PubMed]
- Erdine S, Bilir A, Cosman ER, et al. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract 2009;9:407-17. [Crossref] [PubMed]
- Cosman ER Jr, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med 2014;15:2020-36. [Crossref] [PubMed]


