Self-expanding metal stents in palliative malignant oesophageal dysplasia
Introduction
Despite modern developments in both diagnostic and treatment methods, oesophageal cancer remains the sixth leading cause of cancer-related death worldwide, with the reported 5-year survival rate being less than ten percent (1). Patients usually present late in the course of the disease, with inoperable cancer at the time of diagnosis, due to either local invasion, presence of metastatic disease or both (2,3). The delay in diagnosis is primarily accounted by the late development of symptoms attributed to the distensible properties of the gastrointestinal tract. Patients experience few symptoms until advanced disease or severe luminal narrowing has occurred (4,5). In addition, most oesophageal cancers are detected in elderly patients with severe underlying comorbidities (6,7) and a major cause of morbidity in patients with oesophageal and gastric cardia malignancy remains to be dysphagia along with under nutrition (8,9).
Palliative surgery offers the optimal alleviation for oesophageal obstruction symptoms. However, most of these patients are poor surgical candidates either due to advanced disease or their general health status and underlying comorbidities. Hence, treatment is usually restricted to palliation. The aim of palliative treatment focuses on relieving dysphagia, thereby increasing oral nutritional intake whilst also reducing the risk of aspiration and reflux (1,4,10-12). This minimises hospital stay and improves quality of life.
Today, less invasive non-surgical techniques such as oesophageal stenting are widely used as first line treatment options particularly in the subgroup of patients that are not suitable for surgical intervention. Oesophageal stenting, which was first described over 20 years ago (13), has now become the endoluminal treatment of choice related with immediate relief of symptoms. On the downside, the procedure continues to be associated with high incidence of recurrent dysphagia (23-50%) (11,14,15). Herein, we present an up to date review of the current literature regarding malignant oesophageal stenting. In particular, the authors will detail important factors in patient selection, indications, contraindications, procedural steps, complications and stent options available.
Indications, contraindications and pre-intervention workup
Indications and contraindications
Oesophageal stenting has been used for many years as a palliative treatment option, especially in patients with advanced disease not suitable for curative surgical treatment (16). Although stenting is mainly used to treat unresectable tumours, there are reports of retrievable stent placement in patients with dysphagia secondary to potentially resectable oesophageal cancer. In such cases, the aim of stent placement is to enable increase nutritional intake prior to surgery whilst the tumour is down staged with chemotherapy (17).
Irreversible coagulopathy is a relative contraindication for stenting. In patients with bleeding diathesis, stenting can be performed with the use of fresh frozen plasma and platelet transfusion (4,18). A full list of both indications and contraindications for oesophageal stenting are listed in Table 1.
Full table
Pre-intervention work-up
The treatment pathway for patients with oesophageal malignancies should include a multidisciplinary decision to ensure that the least invasive palliative treatment with the best quality of life is offered. When a patient is referred for oesophageal stenting, a complete analysis of the clinical, radiological and endoscopic investigations is essential to enable both patient and clinician to make an informed discussion, understand the advantages and disadvantages as well as the expected result. It also enables the operative clinician the ability for pre-procedural planning (4,18,19). This pre-intervention workup includes endoscopy (consisting of endoscopic biopsies if possible), endoscopic ultrasound (EUS), thin section computer tomography (CT), fluorodeoxyglucose positron emission tomography (FDG-PET) and fluoroscopic contrast studies.
Upper gastrointestinal endoscopy is an essential part of tumour staging and preliminary assessment. It enables endoscopic biopsies to be obtained and assessment of whether the cancer is either superficial (confined to the submucosa) or advanced (invasion of the muscularis propria and beyond). Endoscopic biopsy provides essential staging information due to the strong correlation between tumour depth and the incidence of lymph node metastases (21). It also provides detailed information of the intraluminal extent of the disease.
Fluoroscopic contrast studies and thin section CT imaging, including multiplanar reconstructions, enable the delineation of the patient’s anatomy, morphological appearances of the stricture or obstruction, assessment of extra luminal spread and evaluation of metastatic deposits. The pre-procedural CT scan should ideally be performed with a combination of both intravenous and oral contrast. For the latter water soluble contrast is recommended over barium, particularly in cases with suspected fistulation, as barium aspiration can cause acute pulmonary inflammation (19).
EUS, CT and FDG-PET have been shown to individually play a distinctive role in the identification of metastatic deposits in patients with oesophageal carcinoma. EUS has a higher staging accuracy for regional lymph node metastasis compared to CT. Accuracy of regional nodal staging is superior for EUS (70-80%) compared to CT (40-73% for pathological mediastinal nodes).The overall staging accuracy of EUS is 85-90%, in comparison to 50-80% with CT. EUS is most sensitive for the detection of regional lymph node metastases whilst CT and FDG-PET are more specific tests. The accuracy of FDG-PET in regional nodal staging ranges from 24-90% (17). The main limitation of FDG-PET for nodal metastatic involvement adjacent to the primary tumour is the poor spatial resolution (approximately 6 mm for a dedicated PET scanner) which therefore decreases sensitivity.
The most significant advantage of FDG-PET over conventional imaging is the detection of distant metastases. Distant metastases have a major impact on patient management as these patients are no longer suitable for surgical resection. FDG-PET has a reported sensitivity of 69-100%, specificity of 84-90% and an accuracy of 84-91% for the assessment of distant metastatic deposits. In comparison, the sensitivity of CT for distant metastases has been reported to be lower (17). FDG-PET scans have also excluded metastatic disease at sites that may have previously considered to be abnormal on conventional CT imaging (22).
Pre-intervention radiological imaging is essential as alternative approaches may be used depending on the location of the abnormality. For example, high level stenosis (i.e., within 2 cm of the proximal oesophageal sphincter and close to the vocal cords) is a relative contraindication to stenting due to the high risk of patient discomfort or risk of other stent related complications (i.e., severe pain, foreign body sensation, migration risk and tissue hyperproliferation) (4,23).
Overall, it is preferable to plan oesophageal stenting following completion of chemotherapy and/or radiotherapy treatment (except in patients presenting with complete or severe dysphagia). This minimizes the risk of stent migration due to decrease in tumour size following treatment. The option of placement of a retrievable stent, as a temporary measure, may be more appropriate. Furthermore, covered retrievable expandable metal oesophageal stent placement and concurrent radiotherapy are associated with reduced complications and re-interventions when compared to permanent oesophageal stents (24).
Oesophageal stenting techniques
Oesophageal stents are inserted through a trans-oral approach using either fluoroscopic guidance, endoscopy or a combination of the above. The fluoroscopy alone approach is easy and quick. However, some endoscopists, prefer the combined use of endoscopic and fluoroscopic guidance especially in hospitals were an established experience radiological service is not available. The procedure is typically performed under conscious sedation with a combination of an opiate (i.e., fentanyl: 50-100 µm) and a sedative (i.e., midazolam: 2-6 mg) (12).
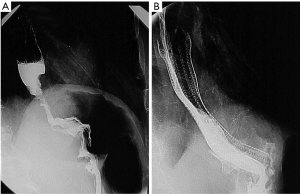
The patient is usually positioned in the left lateral decubitus position as this enables optimal opacification of the gastroesophageal junction (GEJ) (Figure 1A). However, if the patient is unable to tolerate this position, the procedure can be performed in the supine position. A right anterior oblique view enables accurate stent placement at the GEJ. A tilting table allows for the head to be placed in a raised position to decrease the risk of aspiration during the procedure.
Following anaesthesia of the pharynx with 1% xylocaine spray and appropriately titrated sedation, a 6 Fr biliary manipulation catheter (BMC) and a soft wire, such as a Benson wire is used to negotiate through the oesophageal stricture. If this is unsuccessful, the Benson wire can be replaced with a standard angled hydrophilic wire (Terumo, Tokyo, Japan). Using standard techniques both the wire and catheter are directed through the stricture into stomach. It is preferable to advance the wire into the duodenum as this allows better security and safer advancement of the stent introducer system.
The wire is then exchanged for a long stiff 260 cm Amplatz type wire which acts as a support wire for the oesophageal stent. Usually, at this point, especially in cases of obstructive lesions, where the extent of the obstruction is unclear on pre-procedural imaging, the extent of the obstruction can be delineated with the use of a long sheath. The sheath is passed over the stiff wire, past the stricture, the inner dilator is removed over the wire and then contrast is slowly injected through the sheath whilst simultaneously retracting the sheath cranially across the stricture. If deemed necessary, double contrast images can be achieved, with the use of a combination of iodinated contrast and air (21). In this way a detailed analysis of the site, severity and length of the stricture can be recorded allowing for more confident placement of the stent (as the contrast delineates the level of the stricture at the time of the procedure).
In general, a stent that is at least 3-4 cm longer than the stricture is recommended to account for stent shortening and to ensure satisfactory stent coverage across the lesion. This ensures 1-2 cm cephalic and caudal coverage of the lesion (4,19). It is recommended that placement of the stent is performed so that two thirds of the stent is placed cranial (proximal) to the stricture. This is to reduce the possibility of stent migration (4). Oesophageal stricture predilation is not advised due to the risk of perforation but can be performed in cases of tight stenosis to allow stent delivery (18,25,26). In such cases, gentle dilation usually with a 10-14 mm balloon may be performed. In patients with tight strictures requiring pre-dilatation, if dilatation is not performed prior to stent insertion, then it can be done post stenting.
After stent placement, if the stricture is not tight, post dilation is not usually required, as the stent gradually expands to its nominal diameter in 24-48 hours.
Injection of water soluble contrast via a catheter is performed following stent deployment (Figure 1B) to confirm stent patency and exclude the possibility of perforation. However, it is important to note that an initial catheter contrast study may miss a perforation within the non-dependent wall of the oesophagus. Hence, it is advisable to perform a repeat water soluble contrast study four to 12 hours after stenting to confirm accurate stent placement, stent expansion, luminal patency and identification of complications such as iatrogenic perforation. Stenting of the upper oesophagus is a challenge. Several studies have demonstrated it to be safe and easy to perform with virtuous outcomes. However, stenting of this region is proven to be associated with apprehension. Many clinicians have concerns regarding cough, throat discomfort and foreign body sensation. Due to relative lack of experience of upper oesophageal stents, many patients are sentenced to feeding jejunostomies instead. Both dilatation of the upper oesophageal stenotic tumour or endoscopic laser boring are not favoured due to the association with perforation, haemorrhage, aspiration pneumonia and repeated treatments to maintain luminal patency (27).
Stent options
There has been great interest and attention into stent design, mainly driven from the deficiency of improvements in clinical outcomes. Accordingly, identification of factors related to the actual stent design is of great interest. Oesophageal stents consist of stainless steel, nitinol or plastic strut matrix and can be either covered or uncovered. Covered plastic stents have now been superseded by self-expandable metal stents (SEMS) which have revolutionized the treatment of malignant dysphagia patients by providing safe, rapid and effective symptomatic relief (28-32). Stents provide internal splinting of the lumen and radial force to compress against the obstructing tumour. Newer stents are constructed from nitinol. This is more advantageous when compared to older generation stainless steel stents as such devices conform to a predicable self-expanding shape after deployment (11,25,33,34).
There are several oesophageal stents on the market today (Table 2).

Full table
Biodegradeable oesophageal self-expandable stents are the latest commercially available stent options (ELLA-BD stent by ELLA-CS). In these, disintegration occurs within 11-12 weeks’ time period. The absorption is accelerated by reflux of acid gastric contents. In cases of stent migration, the stent can be left to dissolve within the stomach. However, this stent requires hand loading and is not easily visible under fluoroscopy (22).
Covered or uncovered stents?
Oesophageal stents can be covered, uncovered or double type. Both membrane covered and uncovered metal stents provide better palliation and fewer complications compared to plastic prosthetic stents (28). Originally oesophageal stents were uncovered (Figure 2). These stents had the advantage of allowing epithelisation of the stent within 3-6 weeks after which there was no risk of migration. The disadvantage of such stents was tumour in- growth through the interspaces of the metal mesh, resulting in luminal narrowing and recurrent dysphagia (14,19,35). Covered self-expanding stents, in contrast prevent tumour ingrowth thereby preventing oesophageal re-obstruction but are more prone to stent migration secondary to peristalsis and gravitation due to lack of epithelisation. This is particularly problematic in the lower oesophagus (36). Covered stents also require larger delivery systems compared to uncovered stents. Overall migration rates of covered stents have been reported between 5% and 32% (37-41). In contrast, uncovered stents have relatively lower migration rates (0-3%) (28,42-45).
Uncovered stents are particularly useful in cases of extrinsic oesophageal compression, dilated oesophagus and tumour recurrence following gastric pull-up surgery. A very dilated oesophagus can result in food entrapment between the proximal stent and the oesophagus whilst in cases of tumour recurrence in gastric pull-up surgery, uncovered stents minimises stent migration (4).
Covered oesophageal stents are beneficial for the treatment of malignant fistulas such as tracheo-esophageal or broncho-esophageal fistulas as the stent can relieve respiratory distress in this group of patients (46-49). Simultaneous tracheobronchial and oesophageal stenting is useful for the treatment of malignant trachea-esophageal fistulation thereby treating the symptoms of both dysphagia and airway compromise (50-52). Placement of covered retrievable expandable nitinol stent are safe and effective in the treatment of oesophagorespiratory fistulae (53). Temporary insertion of a covered retrievable stent is useful for the treatment of iatrogenic perforation (4,11,46,54,55).
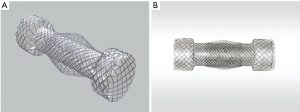
Relatively recently, and in order to encompass the benefits of both the covered (low tumour ingrowth) and uncovered stent designs (low migration) two new type of stents became available (8,56). The Niti-S covered oesophageal stent (Taewoong Medical, Seoul, Korea) consists of a braided nitinol (braided nickel titanium alloy) wire with a polyurethane membrane cover over the entire length of the stent. The covered part of the stent, with a standard diameter of 18 mm, prevents tumour ingrowth (12,37). The stent flares to 26 mm at the proximal and distal ends in a “dog-bone end design” reducing the possibility of migration. Another version is the oesophageal double layered Niti-S stent (Figure 3A and B). In this case the stent has the same basic design of the covered stent but has an additional, uncovered nitinol wire mesh on the outer layer of the body of the stent. These stents enables a more secure anchoring of the body of the stent into the tumour tissue, thereby reducing stent migration even further (56).
Both the covered and double type stents are available in four lengths (6, 8, 10, 12 and 15.5 cm) and are delivered in an 18 Fr introducer sheath. The stent is held, compressed and elongated on the delivery catheter by a cylindrical rolling membrane (the outer sheath). The delivery catheter has three marker positions: two markers at the proximal part of the stent portion (the proximal markers), two in the middle of the stent and two markers at the distal position of the loaded stent (the distal stent marker). Stent deployment is accomplished by withdrawing the outer sheath while fixing the inner catheter. There is stent shortened by approximately 35% from the distal side when it is deployed, whereas the proximal end does not foreshorten (57).
In a prospective, randomised, single centre trial involving 36 consecutive patients with malignant dysphagia due to inoperable oesophageal or gastric cardia cancer, the double type Niti-S covered stent demonstrated less stent related complications, particularly tumour ingrowth and stent migration (47). The use of the double layer configuration stents have been associated with low (less than 7%) migration rates (58).
Stenting across the gastroesophageal junction
There is an increased incidence of tumours involving the distal oesophagus and the gastric cardia. Hence stents are increasingly used across the GEJ. Technically placement of stents across the GEJ are an easy and safe palliative treatment option but there are persistent issues specifically associated with the anatomic location. These include stent migration and reflux oesophagitis (39).
Migration tendencies in this location are due to the distal portion of the stent projecting freely into the gastric fundus without actual fixation to the oesophageal or gastric wall (59). Although today there are several studies reporting the efficacy of stents in malignant obstruction, there are limited data regarding stent insertion across the GEJ (60-62). Migration rates for covered oesophageal stents varies from 7% to 50% in published series (63-67). In contrast, migration rates for double layered stent placement at the GEJ were reported to be as low as 4.7%.
A recent retrospective study conducted by Kim et al. (32) assessed the double layered stent (Nit-S double layered oesophageal stent; Taewoong, Seoul, South Korea) in 48 consecutive patients over a 5-year period which demonstrated a 5.6% (one patient) incidence of stent migration. This study also showed a 27% incidence of symptomatic reflux esophagitis, which is similar to other studies (38,63).
The reason for the development of reflux oesophagitis following stent placement at the GEJ is that a stent in this anatomical position eliminates the physiological sphincter function of the oesophagus and allows free reflux of gastric contents into the oesophagus. Patients therefore are more susceptible to reflux when intra-abdominal pressure is increased or passive reflux when gravity is eliminated (68). Hence, stenting over the GEJ also increases the risk of aspiration and death (69). Proton pump inhibitors are effective for controlling symptomatic gastro-esophageal reflux in the majority of patients (4,70).
A potential solution to the problem of reflux in such patients is the use of a valved stent (71,72). Several anti-reflux stents have been developed and clinically tested over the past few years in order to prevent reflux but so far the results have been non-conclusive. In addition, these stents have been associated with higher migration rates and symptoms of post prandial gas bloating (73). A randomised controlled study by Sabharwal et al. (65) demonstrated no direct benefits of the use of anti-reflux stents in comparison to standard open Ultraflex stents combined with the use of proton pump inhibitors.
Aftercare and nutrition
Stent insertion can be performed as a day case procedure. It is important to warn patients that they may develop temporary chest pain following stent insertion. This usually lasts a few days and is accounted by persistent stent expansion (74) which continues post insertion. During this period, opioid analgesia for pain relief maybe required. Patients should also be alerted to the early or late potential risk of haemorrhage and fistulation.
All patients should be provided with clear post procedural instructions in regards to nutritional intake. Usually, in uneventful oesophageal stent insertion, the patient should remain nil by mouth for four hours. If there are no problems after this period (evidence of perforation or haemorrhage), the patient can then commence initially on free fluids and then progress to a soft, low fibre diet with encouragement to drink copious amounts of water and carbonated drinks (which encourages wash out of food remnants). Patients with oesophageal stents should not lie flat but sleep at a 30 degree angle and should eat in an upright position. All patients should be prescribed a proton pump inhibitor as a prophylaxis to reduce reflux disease following stenting across the GEJ (4,19).
Clinical results of stent placement
Successful treatment of a malignant stricture can be assessed on the basis of either technical success (uncomplicated stent insertion across the obstruction) or clinical success (defined a relief of obstructive symptoms and the oral intake of nutrition following stenting) (18,75). Clinical success in terms of improvement in swallowing can be assessed with the use of the dysphagia score (Table 3). In the majority of patients, the dysphagia score decreases by at least one grade.

Full table
The overall reported technical success rate of oesophageal stenting is very high with prompt relief of dysphagia in up to 96% (53). A recently published retrospective study demonstrated that the technical success of the Nit-S double layered oesophageal covered stent was 95% (76). Clinical success of covered stents in the treatment of malignant oesophageal fistulation to the airway has been reported between 95-100% (77,78).
Stents have been shown to significantly improve symptoms of dysphagia, increase nutritional intake and improve quality of life in palliative patients. This is despite the fact that the majority of patients die within 4-months post stent insertion mainly due to the extent of the disease at the time of diagnosis (79). Burstow et al. reported a median survival of 153 days compared to 72 days after stenting and adjuvant therapy in comparison to stent alone (80). A more recent study by Kofoed et al. (81) concluded that patients who received palliative chemotherapy or chemoradiotherapy in conjunction with stents had a longer median survival than those who were palliated with stents alone. However, it is crucial to note that the presence of an oesophageal stent causes problems with accurate radiotherapy dosimetry. There is currently no definitive conclusive evidence on the increased risks of oesophageal perforation or haemorrhage following chemoradiotherapy (19). Despite this, a 3-6-week interval is usually recommended between chemoradiotherapy and stenting (4).
Interestingly, a retrospective cohort study conducted by Gray et al. (82) demonstrated a correlation between nutritional factors on survival in patients with palliative oesophageal cancer undergoing stent insertion. The study concluded that the requirement for invasive nutritional support before stent insertion was associated with a poor prognosis and this was probably due to more aggressive tumour pathology (81).
Complications
Technical failure (including stent misplacement and deployment failure) is less than 1% (83) (Table 4). Major peri-procedural complication, rate (such as haemorrhage, infection and perforation) is low. In addition, most of these complications are often self-limiting requiring no further interventions. Chest pain is usually related to stent expansion and is reported in 12-14% and is more prevalent in flared stent designs such as the Flamingo stent. Haemorrhage is usually self-limiting and occurs in 3-8%. In conjunction, tumour ingrowth is rare with covered stents compared to bare stents (17-36%). Perforation risk is also low at less than 1% and can be treated with the use of a covered stent or co-axial insertion of another overlapping stent. Procedure related mortality ranges between zero and 1.4% in published series (10,52,84).
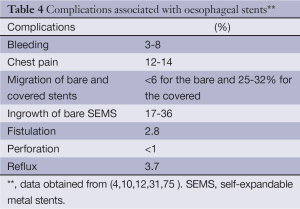
Full table
Migration of bare stents is less common (0-6%) compared to covered stents (25-32%). It can be treated with co-axial insertion of another overlapping stent to ensure the whole length of the original stricture is stented. If the stent has completely migrated and results in obstructive symptoms, then the stent has to be removed either endoscopically or surgically (4).
A recent 3-year retrospective study of 56 patient at a single centre demonstrated that the technical success of the Nit-S double layered oesophageal covered stent was 95% (75). In this study, stent migration (7.1%) exclusively occurred in the group of patients who received chemoradiotherapy as this was secondary to tumour shrinkage.
As part of late complications, tumour ingrowth or overgrowth around an existing stent can be treated with repeat coaxial stent placement (56,83). Other late complications, are often related to the stent itself, include stent fracture and fistulation following oesophageal wall erosion (7,37). Finally, a rare complication that has been reported is the expulsion of an INTACT oesophageal stents with vomiting following complete response to disease after three cycles of chemotherapy (85).
Future advances in oesophageal stenting
Oesophageal stents are constantly evolving with industry continuously modifying stent structure and design. Despite this, the overall complication rate still remains higher than ideal. It has been suggested that applying fibrin glue to the interface of the gastrointestinal wall and the stent or modification of the cranial and caudal part of the stent may decrease the incidence of stent migration (86). In addition, increasing stent flexibility and prevention of membrane separation can be achieved by covering the PTFE membrane externally to the stent wire mesh (87). Drug-eluting stents covered with polymers have been suggested to inhibit growth of malignant tissue ingrowth thereby increasing stent patency (88).
Lately, biodegradeable oesophageal stents, for example woven polydioxanone stents, have been manufactured (89,90). Polydioxanone is a polymer that demonstrates biodegradability, flexibility and shape memory ability. Therefore it appears to be an alternative to nitinol wire. A recently published prospective study involving eleven patients investigated the clinical results of the use of biodegradable oesophageal stents in malignant strictures (80). This study demonstrated 100% procedural technical success rate however early complications occurred in 27% of the cases that resulted in failure to restore nutritional intake. Stent dysfunction occurred in 62.5% of the cases due to local inflammatory reaction and tumour ingrowth and hence four out of the five patients had subsequent placement of a SEMS. The study concluded that biodegradable stents do not offer a clear benefit in malignant strictures especially due to a local inflammatory reaction that is induced. However, further improvements are necessary in regards to the stent design as the current biodegradable stents are not radiopaque (as the polymers are radiolucent) and can only be detected by indirect methods. In addition, the radial force and elasticity decreases gradually over time (89,90).
Conclusions
Oesophageal carcinoma continues to account as a major cause of cancer –related deaths worldwide. Stents are now considered an established treatment option for palliation of dysphagia in oesophageal malignancy. Alterative treatment options to esophageal stenting include chemoradiotherapy (91,92), thermal laser ablation (15,93), photodynamic therapy (94,95), or nasogastric tube feeding or gastrostomy placement (96). Unfortunately there is a lack of prospective randomised large control trials investigating the performance of stents compared to alternative options.
The palliative treatment of esophageal carcinoma is challenging and the optimal stent is still debated (97). Previous covered plastic stents have now been replaced with metal stents. These can be safely inserted with fewer complications and performed as an outpatient procedure. This has resulted in significant shorter hospital stay and in turn, lower costs (28). The newer double stent design incorporates the benefits of both the uncovered (low migrations) and the covered design (low tumour ingrowth) for the palliative treatment of malignant dysphagia (8,56). Such stents have been crucial in reducing migration rates. Drug-eluting stents covered with polymers may be a key feature in future stent designs by inhibiting growth of malignant tissue ingrowth thereby increasing stent patency (88). Future development of stent design may hold the key to decreasing complications associated with oesophageal stents.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [PubMed]
- Stein HJ, Siewert JR. Improved prognosis of resected oesophageal cancer. World J Surg 2004;28:520-5. [PubMed]
- Blot WJ. Eosphageal cancer trends and risk factors. Semin Oncol 1994;21:403-10. [PubMed]
- Sabharwal T, Morale JP, Irani FG, Adam A. Quality improvement guidelines for placement of esophageal stents. Cardiovasc Intervent Radiol 2005;28:284-8. [PubMed]
- Kubba AK, Krasner N. An update in the palliative management of malignant dysphagia. Eur J Surg Oncol 2000;26:116-29. [PubMed]
- Burstein HJ, Mayer RJ. Gastrointestinal cancer. In: Hunter CP, Johnson KA, Muss HD. eds. Cancer in the elderly 2000. Marcel Dekker Inc., New York, 325-8.
- Katsanos K, Ahmad F, Dourado R, et al. Interventional radiology in the elderly. Clin Interv Aging 2009;4:1-15. [PubMed]
- Riccardi D, Allen K. Nutritional management of patients with esophageal and esophagogastric junction cancer. Cancer Control 1999;6:64-72. [PubMed]
- Kim ES, Jeon SW, Park SY, et al. Comparison of double-layered and covered Niti-S stents for palliation of malignant dysphagia. J Gastroenterol Hepatol 2009;24:114-9. [PubMed]
- Lee SH. The role of oesophageal stenting in the non-surgical management of oesophageal strictures. Br J Radiol 2001;74:891-900. [PubMed]
- Lowe AS, Sheridan MB. Esophageal stenting. Semin Intervent Radiol 2004;21:157-66. [PubMed]
- Morgan R, Adam A. The radiologist’s view of expandable metallic stents for malignant esophageal obstruction. Gastrointest Endosc Clin N Am 1999;9:431-5. [PubMed]
- Domschke W, Foerster EC, Matek W, et al. Self- expanding mesh stent for esophageal cancer stenosis. Endoscopy 1990;22:134-6. [PubMed]
- Song HY, Jung HY, Park SI, et al. Covered retrievable expandible nitinol stents in patients with benign oesophageal strictures: initial experience. Radiology 2000;217:551-7. [PubMed]
- Adam A, Ellul J, Watkinson AF, et al. Palliation of inoperable esophageal carcinoma: a prospective randomised trial of laser therapy and stent placement. Radiology 1997;202:344-8. [PubMed]
- Symonds CJ. A case of malignant stricture of the oesophagus illustrating the use of a new form of oesophageal catheter. Trans Clin Soc Lond 1885;18:155-8.
- Pellen MGC, Sabri S, Razack A, et al. Safety and efficacy of self-expanding removal metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Diseases of the Esophagus 2012;25:48-53. [PubMed]
- Sabharwal T, Irani FG, Adam A, et al. Quality assurance guidelines for placement of gastroduodenal stents. Cardiovasc Intervent Radiol 2007;30:1-5. [PubMed]
- Baerlocher MO, Asch MR, Dixon P, et al. Interdisciplinary Canadian guidelines on the use of metal stents in the gastrointestinal tract for oncological indications. Can Assoc Radiol J 2008;59:107-22. [PubMed]
- Katsanos K, Sabharwal T, Adam A. Stenting of the upper gastrointestinal tract: Current Status. Cardiovasc Intervent Radiol 2010;33:690-705. [PubMed]
- Stivaros SM, Williams LR, Senger C, et al. Woven polydioxanone biodegrade=able stents: a new treatment option for benign and malignant oesophageal strictures. Eur Radiol 2010;20:1069-72. [PubMed]
- Line BR, Maragh MR, Ahamed TB. Positron Emission Tomography Imaging of Lung and Esophageal Cancer. Appl Radiol 2003;31:9-17.
- Choi EK, Song HY, Kim JH, et al. Covered metallic stent placement in the management of cervical oesophageal strictures. J Vasc Interv Radiol 2007;18:888-95. [PubMed]
- Shin JH, Song HY, Kim JH, et al. Comparison of temporary and permanent stent placement with concurrent radiation therapy in patients with esophageal carcinoma. J Vasc Interv Radiol 2005;16:67-74. [PubMed]
- Lopera JE, Brazzini A, Gonzales A, et al. Gastroduodenal stent placement: current stent placement: current status. Radiographics 2004;24:1561-73. [PubMed]
- Zollikofer CL, Jost R, Schoch E, et al. Gastrointestinal stenting. Eur Radiol 2000;10:329-41. [PubMed]
- Yim HB. Stenting of malignant upper oesophageal obstruction: experience in a regional hospital in Singapore. Eur J Gastroenterol Hepatol 2011;23:948-51. [PubMed]
- Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of oesophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302-7. [PubMed]
- Ell C, May A. Self-expanding metal stents for palliation of stenosing tumours of the oesophagus and cardia: a critical review. Endoscopy 1997;29:392-8. [PubMed]
- Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc 1998;48:172-9. [PubMed]
- Baron TH, Schöfl R, Puespoek A, et al. Expandable metal stent placement for gastric outlet obstruction. Endoscopy 2001;33:623-8. [PubMed]
- Kim MD, Park SU, Kang DH, et al. Double layered self-expanding metal stents for malignant esophageal obstruction, especially across the gastroesophageal junction. World Journal of Gastroenterology 2012;18:3732-7. [PubMed]
- Simmons DT, Baron TH. Technology insight: enteral stenting and new technology. Nat Clin Pract Gastroenterol Hepatol 2005;2:365-74. [PubMed]
- Siersema PD. Treatment options for oesophageal strictures. Nat Clin Pract Gastroenterol Hepatol 2008;5:142-52. [PubMed]
- Vakil N, Gross U. Bethge. Human tissue responses to metal stents. Gastrointest Endosc Clin N Am 1999;9:359-65. [PubMed]
- Sabharwal T, Hamady MS, Chui S, et al. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut 2003;52:922-6. [PubMed]
- Wang MQ, Sze DY, Wang ZP, et al. Delayed complications after oesophageal stent placement for treatment of malignant oesophageal obstructions and oesophagorespiratory fistulas. J Vasc Interv Radiol 2001;12:465-74. [PubMed]
- Song HY, Do YS, Han YM, et al. Covered, expandable esophageal metallic stent tubes: experiences in 119 patients. Radiology 1994;193:689-95. [PubMed]
- Song HY, Lee DH, Seo TS, et al. Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures. J Vasc Interv Radiol 2002;13:285-93. [PubMed]
- Miyayama S, Matsui O, Kadoya M, et al. Malignant esophageal stricture and fistula: palliative treatment with polyurethane-covered Gianturco stent. J Vasc Interv Radiol 1995;6:243-8. [PubMed]
- Saxon RR, Morrison KE, Lakin PC, et al. Malignant esophageal obstruction and esophagorespiratory fistula: palliation with a polyethylene-covered Z stent. Radiology 1997;202:349-54. [PubMed]
- Cwikiel W, Tranberg KG, Cwikiel M, et al. Malignant dysphagia: palliation with esophageal stents- long term results in 100 patients. Radiology 1998;207:513-8. [PubMed]
- Cwikiel W, Stridbeck H, Tranberg KG, et al. Malignant esophageal strictures: treatment with a self-expanding nitinol stent. Radiology 1993;187:661-5. [PubMed]
- Acunaş B, Rozanes I, Akpinar S, et al. Palliation of malignant esophageal strictures with self-expanding nitinol stents: drawbacks and complications. Radiology 1996;199:648-52. [PubMed]
- Winkelbauer FW, Schöfl R, Niederle B, et al. Palliative treatment of obstructing esophageal cancer with nitinol stents: value, safety and long term results. AJR Am J Roentgenol 1996;166:79-84. [PubMed]
- Saxon RR, Morrison KE, Lakin PC, et al. Malignant esophageal obstruction and esophagorespiratory fistula: palliation with a polyethylene-covered Z stent. Radiology 1997;202:349-54. [PubMed]
- Dumonceau JM, Cremer M, Lalmand B, et al. Esophageal fistula sealing: choice of stent, practical management and cost. Gastrointest Endosc 1999;49:70-8. [PubMed]
- Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc 1998;48:172-9. [PubMed]
- Sihoe AD, Wan IY, Yim AP. Airway stenting for unresectable esophageal cancer. Surg Oncol 2004;13:17-25. [PubMed]
- Yamamoto R, Tada H, Kishi A, et al. Double stent for malignant combined esophago-airway lesions. Jpn J Thorac Cardiovasc Surg 2002;50:1-5. [PubMed]
- Ellul JP, Morgan R, Gold D, et al. Parallel self-expanding covered metal stents in the trachea and oesophagus for the palliation of complex high trachea-oesphageal fistula. Br J Surg 1996;83:1767-8. [PubMed]
- Katsanos K, Sabharwal T, Koletsis E, et al. Direct erosion and prolapse of oesophageal stents into the tracheobronchial tree leading to life-threatening airway compromise. J Vasc Interv Radiol 2009;20:1491-5. [PubMed]
- Lee KE, Shin JH, Song HY, et al. Management of airway involvement of oesophageal cancer using covered retrievable nitinol stents. Clin Radiol 2009;64:133-41. [PubMed]
- Sabharwal T, Morale JP, Salter R, et al. Esophageal cancer: self-expanding metallic stents. Abdom Imaging 2005;30:456-64. [PubMed]
- Kim KR, Shin JH, Song HY, et al. Palliative treatment of malignant esophagopulmonary fistulas with covered expandable metallic stents. AJR Am J Roentgenol 2009;193:W278-82. [PubMed]
- Verschuur EM, Homs MY, Steyerberg EW, et al. A new esophageal stent design (Niti-S stent) for the prevention of migration: a prospective study in 42 patients. Gastrointest Endosc 2006;63:134-40. [PubMed]
- Fujita T, Tanabe M, Shimizu K, et al. Radiological Image- Guided Placement of Covered Niti-S Stent for palliation of dysphagia in patients with cervical esophageal cancer. Dysphagia 2013;28:253-9. [PubMed]
- Park JG, Jung GS, Oh KS, et al. Double-layered PTFE- Covered nitinol stents: experience in 32 patients with malignant esophageal strictures. Cardiovasc Intervent Radiol 2010;33:772-9. [PubMed]
- Siersema PD, Marcon N, Vakil N. Metal stents for tumours of the distal oephagus and gastric cardia. Endoscopy 2003;35:79-85. [PubMed]
- Scheithauer W. Esophageal cancer: chemotherapy as palliative therapy. Ann Oncol 2004;15:Suppl 4: iv 97-i100.
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005;10 Suppl 3:49-58. [PubMed]
- Hung AY, Canning CA, Patel KM, et al. Radiation therapy for gastrointestinal cancer. Hematol Oncol Clin North Am 2006;20:287-320. [PubMed]
- Vakil N, Morris AI, Marcon N, et al. A prospective, randomised, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol 2001;96:1791-6. [PubMed]
- Park JJ, Lee YC, Kim BK, et al. Long-term clinical outcomes of self-expanding metal stents for treatment of malignant gastroesophageal junction obstructions and prognostic factors for stent patency: effects of anticancer treatments. Dig Liver Dis 2010;42:436-40. [PubMed]
- Sabharwal T, Gulati MS, Fotiadis N, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol 2008;23:723-8. [PubMed]
- Christie NA, Buenaventura PO, Fernando HC, et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: short-term and long-term follow-up. Ann Thorac Surg 2001;71:1797-801; discussion 1801-2.
- Homs MY, Steyerberg EW, Kuipers EJ, et al. Causes and treatment of recurrent dysphagia after self-expanding metal stent placement for palliation of oesophageal carcinoma. Endoscopy 2004;36:880-6. [PubMed]
- Dua KS, Kozarek R, Kim J. Self-expanding metal esophageal stent with anti-reflux mechanism. Gastrointest Endosc 2001;53:603-13. [PubMed]
- Valbuena J. Palliation of gastroesophageal carcinoma with endoscopic insertion of a new antireflux prosthesis. Gastrointest Endosc 1984;30:241-3. [PubMed]
- Baron TH. Minimizing endoscopic complications: endoluminal stents. Gastrointest Endosc Clin N Am 2007;17:83-104. [PubMed]
- Dua KS, Kozarek R, Kim J, et al. Self-expanding metal esophageal stent with anti-reflux mechanism. Gastrointest Endosc 2001;53:603-13. [PubMed]
- Köcher M, Dlouhy M, Neoral C, et al. Esophageal stent with antireflux valve for tumors involving the cardia: work in progress. J Vasc Interv Radiol 1998;9:1007-10. [PubMed]
- Schoppmeyer K, Golsong J, Schiefke I, et al. Antireflux stents for palliation of malignant esophagocardial stenosis. Dis Esophagus 2007;20:89-93. [PubMed]
- Golder M, Tekkis PP, Kennedy C, et al. Chest pain following oesophageal stenting for malignant dysphagia. Clin Radiol 2001;56:202-5. [PubMed]
- Dormann A, Meisner S, Verin N, et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy 2004;36:543-50. [PubMed]
- Mezes P, Krokidis ME, Katsanos K, et al. Palliation of Esophageal Cancer with a Double-layered Covered Nitinol Stent: Long-term Outcomes and Predictors of Stent Migration and Patient Survival. Cardiovasc Intervent Radiol 2014. [Epub ahead of print].
- Morgan RA, Ellul JP, Denton ER, et al. Malignant oesophageal fistulas and perforations: management with plastic covered metallic endoprostheses. Radiology 1997;204:527-32. [PubMed]
- Nicholson AA, Royston CM, Wedgewood K, et al. Palliation of malignant oesophageal perforation and proximal oesophageal malignant dysphagia with covered metal stents. Clin Radiol 1995;50:11-14. [PubMed]
- Madhusudhan C, Saluja SS, Pal S, et al. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: impact on quality of life. Dis Esophagus 2009;22:331-6. [PubMed]
- Burstow M, Kelly T, Panchani S, et al. Outcomes of palliative esophageal stenting for malignant dysphagia: a retrospective analysis. Dis Esophagus 2009;22:519-25. [PubMed]
- Kofoed SC, Lundsgaard M, Ellemann AC, et al. Low morbidity after palliation of obstructing gastro-oesophageal adenocarcinoma to restore swallowing function. Dan Med J 2012;59:A4434. [PubMed]
- Gray RT, O’Donnell ME, Scott RD, et al. Impact of nutritional factors on survival in patients with inoperable oesophageal cancer undergoing self-expanding metal stent insertion. Eur J Gastroenterol Hepatol 2011;23:455-60. [PubMed]
- Ramirez FC, Dennert B, Zierer ST, et al. Esophageal self-expandable metallic stents--indications, practice, techniques, and complications: results of a national survey. Gastrointest Endosc 1997;45:360-4. [PubMed]
- Watson A. Self-expanding metal oesophageal endoprostheses: which is best? Eur J Gastroenterol Hepatol 1998;10:363-5. [PubMed]
- Krokidis M, Burke C, Spiliopoulos G, et al. The use of biodegradable stents in malignant oesophageal strictures for the treatment of dysphagia before neoadjuvant treatment or radical radiotherapy: a feasibility study. Cardiovasc Intervent Radiol 2013;36:1047-54. [PubMed]
- Almeida MJ, Yoshida WB, Hafner L, et al. Biomechanical and histologic analysis in aortic endoprosthesis using fibrin glue. J Vasc Surg 2011;53:1368-74. [PubMed]
- Na HK, Song HY, Yeo HJ, et al. Retrospective comparison of internally and externally covered retrievable stent placement for patients with benign urethral strictures caused by traumatic injury. AJR Am J Roentgenol 2012;198:W55-61. [PubMed]
- Guo Q, Guo S, Wang Z. A type of esophageal stent coating composed of one 5 flurouracil-containing EVA layer and one drug free protective layer: in vitro release, permeation and mechanical properties. J Control Release 2007;118:318-24. [PubMed]
- Stivaros SM, Williams LR, Senger C, et al. Woven polydioxanone biodegradable stents: a new treatment option for benign and malignant oesophageal strictures. Eur Radiol 2010;20:1069-72. [PubMed]
- Janík V, Horák L, Hnaníček J, et al. Biodegradable polydioxanone stents: a new option for therapy-resistant anastomotic strictures of the colon. Eur Radiol 2011;21:1956-61. [PubMed]
- Earlam R, Cunha-Melo JR. Oesophogeal squamous cell carcinoms: II. A critical view of radiotherapy. Br J Surg 1980;67:457-61. [PubMed]
- Highley MS, Parnis FX, Trotter GA, et al. Combination chemotherapy with epirubicin, cisplatin and 5-fluorouracil for the palliation of advanced gastric and oesophageal adenocarcinoma. Br J Surg 1994;81:1763-5. [PubMed]
- Tan BS, Mason RC, Adam A. Minimally invasive therapy for advanced oesophageal malignancy. Clin Radiol 1996;51:828-36. [PubMed]
- Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfirmer sodium versus thermal ablation therapy with ND: YAG laser for palliation of esophageal cancer: a multicentre randomised trial. Gastrointest Endosc 1995;42:507-12. [PubMed]
- Chen M, Pennathur A, Luketich JD. Role of photodynamic therapy in unresectable esophageal and lung cancer. Lasers Surg Med 2006;38:396-402. [PubMed]
- Mekhail TM, Adelstein DJ, Rybicki LA, et al. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer 2001;91:1785-90. [PubMed]
- Schweigert M, Dubecz A, Stein HJ. Oesophageal cancer--an overview. Nat Rev Gastroenterol Hepatol 2013;10:230-44. [PubMed]