Current characteristics on small intestinal stromal tumor—a case control study
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the gastrointestinal tract. GIST was firstly put forward by Mazur and Clark in 1983 (1), it accounts for less than 1% of the tumors in gastrointestinal tract. GIST can occur in any part of the gastrointestinal tract and the abdomen, stomach is the most common place (60%), followed by small intestine (30%), colorectal (10%), esophageal (0-6%), rarely in omentum and retroperitoneal (2).
Small intestine is the second most popular location of GIST, which is named small intestinal stromal tumor (SIST). The cumulative incidence of malignancy of SIST is twice that of gastric GIST (2). The most common clinical manifestations of SIST are gastrointestinal bleeding, intestinal obstruction, perforation or change in defecation habit. Because the small intestine is up to 5-6 meters in length, overlapped with each other, and the location anatomy is not fixed with large mobility, SIST is easy to be missed diagnosed in clinical work. Nowadays with the development of multislice CT, endoscopic technology and development of molecular biology, the diagnosis and treatment of SIST have been obviously improved. The main treatment of SIST is surgical resection, and the molecular targeted drugs such as imatinib and sunitinib also have made remarkable curative effect on SIST patients. However, research studies on SIST are relatively rare, the present study aims to analyze the clinical manifestations, histopathological and immunohistochemical features, advantages and disadvantages of various auxiliary examination, the treatment and prognosis of SIST, in order to improve our understanding of this disease and provide valid statistics for clinical diagnosis and treatment.
Methods
This retrospective study included 75 patients with SIST who underwent surgery resection and postoperative pathological diagnosis from January 2012 to December 2017 in Xijing hospital. The characteristics of the patients, such as incidence age, gender, clinical manifestation, incidence location, tumor size, the gastrointestinal endoscopic results, image results, operation data and postoperative pathological data were collected and analyzed.
The inclusion criteria were (I) patients’ age should be above 18 years old; (II) patients should have undergone surgical treatment (including open and laparoscopic surgery); (III) patients should be diagnosed as SIST by pathological and immunohistochemical methods or genetic mutation detection (Observation of spindle cells under the microscope, Immunohistochemical analysis of CD117 positive cells or KIT/PDGFRA gene mutation detection confirmed by senior pathologist); (IV) data of cases were accessed completely; (V) patients should not underwent treatment such as chemotherapy, radiotherapy and imatinib therapy. The exclusion criteria were (I) pregnant and lactating women were excluded; (II) patients diagnosed as GIST and other malignant tumor patients should be excluded; (III) patients with serious diseases, which may interfere with the evaluation of this study were excluded; (IV) during the study period, 8 patients were excluded: 2 patients were excluded due to cardiovascular diseases, 2 cases were excluded because they were unable to be contacted due to the far distance, 3 cases were excluded because they were unable to bear the follow-up examination and treatment due to the economy, 1 patient with asthma was excluded because he cannot tolerate the post-examination and treatment. Another 4 patients were excluded because they were unable to be contacted due to the far distance or they changed their telephone numbers. In total, 12 patients were excluded at the end of the study.
The diagnostic standards of SIST
Pathological standard
In this study, the pathologic specimens were all from operation excision, diagnosis was made not only by pathologic specimens, but also imaging results and endoscopic results. Pathological diagnostic standards were referenced to the 2013 edition Chinese expert committee consensus on GIST.
Biological evaluation standard
The present study showed that there was no absolute benign tumor in GIST. In 2008, Joensuu (3) revised the principle for the risk classification of postoperative primary GIST according to the health risk classification system of the United States national institutes of health (NIH).
Definition of the outcome of patients
According to previous studies, patients’ outcomes were divided into favorable outcome and adverse outcome. Favorable outcome was defined as the patients had no recurrence, metastasis and death within 5 years after operation. While adverse outcome means the patients had recurrence, metastasis and death within 5 years after operation (3-5).
Statistical analysis
All statistical analyses were conducted using SPSS software (version 22.0, Chicago, Illinois, USA). A Chi-square or Fisher’s test was used for categorical variables. Continuous variables were compared with Student's t test. If the test of homogeneity of variances between the groups was significant, the Mann-Whitney U test was adopted as appropriate. ROC curves were used to assess the feasibility of using the maximum diameter of the tumor as a predictive tool for patient prognosis. Youden index was used for the evaluation of the optimal cut-off point. The independent predictors for the patient prognosis were calculated using the Cox regression model. Two-tailed P values <0.05 were considered to be statistically significant.
Results
General statistics
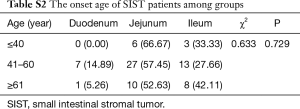
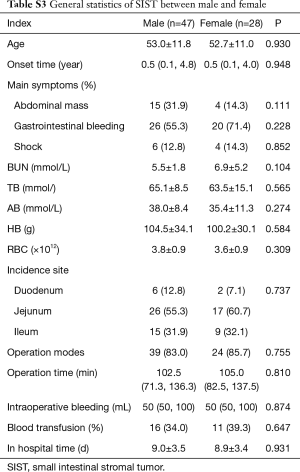
There was no statistical difference in gender distribution, age, onset time, main symptoms, biochemical index, operation modes, operation time, intraoperative bleeding, blood transfusion and in hospital time (P>0.05), as shown in (Tables S1-S3).

Full table
Full table
Full table
Clinical symptoms
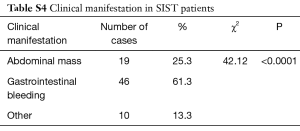
In the present study, 19 cases had abdominal mass, a total of 46 cases had gastrointestinal bleeding, 30 cases had black stool, 2 cases had hematemesis with black stool, 14 cases had dark red stool and among which 10 patients had hemorrhagic shock. The number of patients who had gastrointestinal bleeding was significantly higher than patients who had abdominal mass (P<0.05) (Table S4).
Full table
The primary incidence site
Eight of 75 patients (10.7%) had SIST in the duodenum, 43 patients (57.3%) in the jejunum and 24 patients (32.0%) in the ileum. Cases in the jejunum were significantly more than that in the other groups (P<0.001) (Table S5).
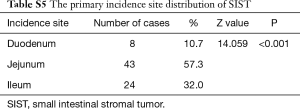
Full table
Imaging and gastrointestinal endoscopy examination
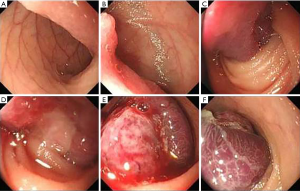
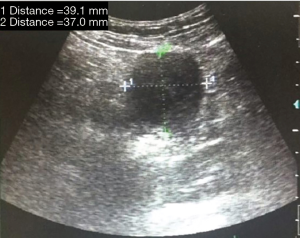
Seventy-three of 75 (97.3%) patients underwent ultrasound examination, with the positive rate 30.1% (22/73). 60 of 75 (80.0%) patients underwent gastrointestinal endoscopy, with the positive rate 23.3% (14/60). Representative figures of SIST under the detection of ultrasound or enteroscopy were shown in Figures 1,S1.
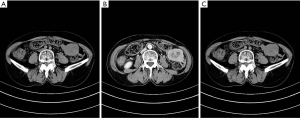
Preoperatively 71 patients underwent small intestine dual-source CT (DSCT) check, with the positive rate 87.3% (62/71). 42 patients (59.2%) had jejunum stromal tumor, 22 patients (31.0%) had ileum stromal tumor, 7 patients (9.9%) had duodenal stromal tumor, with the minimum one 0.9 cm in diameter, the maximum one 20 cm in diameter. 38 cases had tumor <5 cm in diameter, 33 cases had tumor 5 cm or more in diameter and with high degree of malignancy. Representative figures of jejunum stromal tumor under DSCT are shown in Figure 2.
Tumor sizes were measured using the longest diameter of the mass under DSCT. The results are shown in Table 1. Interestingly, from the results of this study, we can conclude that with the increase of tumor diameter, the invasion risk also gradually increased (P<0.001).
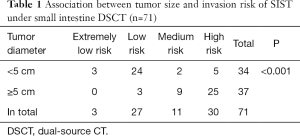
Full table
Histological and immunohistochemical results
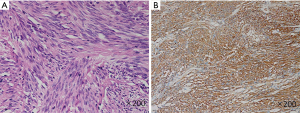
Morphologically, 73 of 75 cases were spindle cell type, representative figures were shown in Figure S2A, 2 cases were spindle-epithelial cell type. All of the 75 cases were tyrosine receptor CD117 positive, representative figures were shown in Figure S2B, 74 of 75 cases were DOG-1 positive, 47 of 75 cases were hematopoietic stem cell antigen CD34 positive.
Surgical statistics
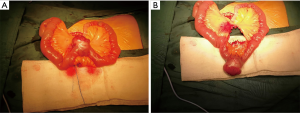
All of the 75 cases underwent surgery, 63 cases (84.0%) had elective surgery, 12 cases (16.0%) had emergency surgery, 8 cases (10.7%) had laparoscopic surgery. 18 cases grew intra cavitary, 37 cases grew extra cavitary (Figure S3), 19 cases grew intra and extra cavitary. Compared to the patients who had elective surgery, patients who underwent emergency surgery mainly manifested shock and gastrointestinal bleeding as shown in Table S6 (P<0.05).
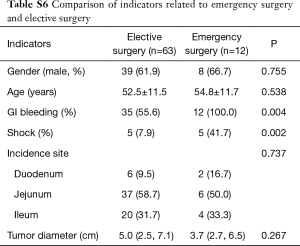
Full table
Single factor analysis of the related risk factors for adverse outcome
Of the 63 cases who were postoperatively followed-up, patients were divided into two groups: favorable outcome group (postoperatively no recurrence, metastasis or death), and adverse outcome group (postoperatively with recurrence, metastasis or death). Patients with adverse outcome had bigger tumor diameter than patients with favorable outcome (P<0.05). For patients with adverse outcome, the nuclear division > 5/50 HPF constitution is significantly higher than patients with favorable outcome (P<0.05). Interestingly, when categorized into 3 cell types according to cell morphology, the spindle-epithelioid cell type appeared only in patients with adverse outcome (P<0.05) (Table 2).
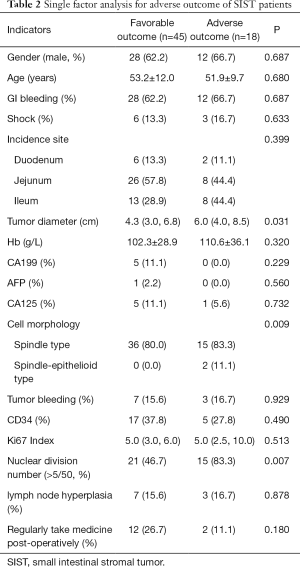
Full table
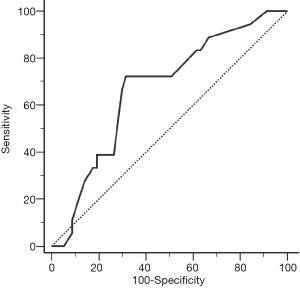
ROC analysis for tumor diameter prediction of postoperative adverse outcome
From the present study, tumor diameter 5.3 cm or higher can predict the postoperative adverse outcome of SIST patients, with the sensitivity 72.2%, specificity 68.4%, and the area under the curve was 0.669 (P=0.032) (Figure 3).
Multi-variate analysis of SIST related risk factors for postoperative adverse outcome
Cox regression analysis indicated that tumor diameter 5.3 cm or higher and nuclear division >5/50 can be independent risk factors for predicting SIST postoperative adverse outcome (Table 3).

Full table
Discussion
GIST usually originates from gastrointestinal mesenchymal tissues and is relatively rare compared to other types of GI tumors. The main symptoms of GIST include nausea, vomiting, abdominal discomfort, abdominal pain, abdominal mass, black stool and anemia. GIST can occur in any part of the gastrointestinal tract, mostly in the stomach (50–70%) and the small intestine (35%) (6). It was reported that in recent years, SIST, as a part of GIST, has become more common, and has more aggressive biological behaviors. In addition, the prognosis of SIST is worse than stomach GIST, and the recurrence rate of SIST is higher than GIST in other parts of the gastrointestinal tract (7,8). SIST are always concealed lesions, lack of characteristic clinical manifestations, and without any symptoms in the early stages. Thus, the early diagnosis of SIST is difficult and the prognosis is different. Because examination methods of the small intestine such as CT, capsule endoscopy and enteroscopy are rarely used as routine examination methods, the early detection rate of SIST is lower than GIST, and SIST is easier to cause missed diagnosis and misdiagnosis.
SIST mainly occurs in the elderly population and is rare in people under the age of 35, however, the younger the patients, the higher possibility that the lesion is malignant. Most of the published literature did not show clear preference of the gender (9,10), some studies showed that male patients were more than female (10,11). In the present study, we also found there were more male patients than female patients, however, there was no statistical difference between the two groups. The average onset age was 54. Other studies have reported the average onset age was 50 to 55 years old, which was similar to the results of this study (12). The incidence sites were mainly in the jejunum (43 cases 58.1%), followed by the ileum (24 cases 32.45%) and duodenal (8 cases 10.8%), which was consistent with the previously studies (9).
There are some differences between SIST and gastric stromal tumor (GIST) (13-20):
- The incidence rate of SIST is lower. Patients are more likely to be misdiagnosed than GIST, because patients lack the typical symptoms and signs at the early stage;
- GIST can be easily diagnosed by gastroscopy, the following biopsy and pathological examination. While the diagnosis of SIST is limited because double balloon enteroscopy is not quite popular and the examination process is cumbersome;
- GIST patients rarely have lymph node metastasis compared with SIST patients. There are about 10~15% SIST patients might have lymph node metastasis;
- The malignant degree of GIST is lower than that of SIST, which are easier to relapse and metastasize after operation;
- The incidence rate of GIST accounts for 40-60% of the digestive tract, which is significantly higher than that of SIST (about 30% to 35%).
SIST lesions are usually conceal, and lack of specified clinical manifestations and early symptoms. Studies have reported the SIST patients often manifested as gastrointestinal bleeding, intestinal obstruction, and intestinal perforation (9). Zang et al. (21) used laparoscopic surgery for the treatment of patients with small intestinal bleeding, and found stroma tumor was the most common reason for intestinal bleeding (62.3%). However, patients can be asymptomatic, especially in cases with smaller tumor size (8,22). The lack of specific clinical manifestations and the small intestine location makes SIST relatively difficult to diagnose. It was reported by other studies that abdominal pain (35.5%) was the most common symptom of SIST, and uncontrolled hemorrhagic shock (6.4%) was less common (23). However, the present study showed that gastrointestinal bleeding was the most severe and common symptom, with the lowest hemoglobin 35 g/L and 10 cases (13.3%) had uncontrolled hemorrhagic shock. Miettinen et al. (13) described the clinical manifestations of 622 patients, and found 256 cases (42%) had gastrointestinal bleeding, which was the main symptom and also consistent with the present study. According to this study, it is vital to timely and accurately diagnose where the bleeding source is, because this kind of hemorrhage can be urgent and lead to death. The gastrointestinal bleeding of SIST usually manifested as repeated, intermittent hematochezia and black stool. The reason for gastrointestinal bleeding might be various, one reason is the blood supply of stromal tumor is rich, and the mucosa easily form ulcer and underwent concurrent hemorrhage (2). Another reason might be the stroma tumor had less stromal collagen, and the vascular wall was thin, which can easily cause bleeding (24).
The present study used imaging and endoscopic checks such as abdominal ultrasound, gastroscopy, colonoscopy, capsule endoscopy, enteroscopy and small intestine DSCT and found the small intestine DSCT detection had the highest detection rate (87.3%). CT scan was reported to be the primary choice for the diagnosis of GIST (25,26). Under CT detection, SIST characterizes as bigger than 10 cm in diameter, calcification, irregular edge, unevenly strengthening, lobulated, ulcers and local lymph node enlargement or even metastasis (7). Most GISTs manifest as >5 cm extra cavitary lesions, with good demarcation, lobulation, necrosis or center hemorrhage, but no calcification (27,28). CT examination can clearly see SIST location, size, shape and density, as well as the relationship of the lesion with the surrounding tissues, such as extrusion, adhesion and invasion. CT also can show what is inside the mass such as cyst, hemorrhage and necrosis. It was reported that Multi-Detector-Row Computed Tomography (MDCT) with small bowel imaging can find lesions earlier than ordinary enhanced CT.
The final diagnosis of SIST relies on the pathological examination. Pathological and immunohistochemical staining showed that the origin of GIST was from c-kit gene mutation on chromosome 11, which leads to its coding protein CD117 over-expressed at a cellular level, therefore, CD117 was regarded as the most characteristic marker of GIST, with the common positive rate 85–100% (29,30). In the present study, all the patients were CD117 positive, which was similar to previous reports. CD34 was one of bone marrow hematopoietic progenitor cell markers, the expression rate of CD34 in GIST was about 60%. Most spindle cell type GIST (especially in the stomach) expresses CD34, but in the small intestine, its expression can be negative in SIST. However, the present study showed 7 of 75 cases were CD34 positive, which was consistent with previous reports that CD34 can be positive in part of small intestine SIST (24). In recent years, the diagnostic value of DOG1 in GIST has been gradually improved, DOG1 is a kind of monoclonal antibody which blocks GIST expression. DOG1 does not only exist in GIST, it also can be positive in uterus, retroperitoneal leiomyoma and leiomyosarcoma. Some studies showed DOG1 might have a potential prognostic impact for GIST (31,32), the present study showed that 74 of 75 cases were DOG-1 positive.
It was agreed by previous reports that surgical resection was the main method for SIST therapy, the final target was RO resection (there was no tumor cells at the edge of the cutting lesions). The tumors themselves were soft and fragile, and easy to be ruptured, thus it is vital to completely cut the lesions in order to avoid abdominal spread (33). Although incidence of SIST ranked the second in GI stroma tumors, the incidence site makes the stroma tumor much more malignant than other parts of the GI tract. Thus, for tumors greater than 5 cm in diameter, surgeons should cut the edge for up to 10 cm. For tumors less than 5 cm in diameter, if the coating is complete without bleeding necrosis, surgeons can appropriately reduce the cut edge distance. According to previous reports, after complete surgical removal, the five-year survival rate of SIST was about 48 to 65% (6). Tabrizian et al. (34) analyzed 26 cases, the median follow-up time was 56.4 months (range, 0.1 to 162.4 months), the 10-year overall survival rate and DFS rate were 91.3% and 71.6% respectively. Some reports also have considered laparoscopic resection as a safe and useful method instead of open resection (35,36). It was reported laparoscopic surgery was suitable for patients with tumor diameter less than 5 cm. For lesions located at jejunum and ileum, laparoscopic surgery could be a better choice to find the lesions (12,15).
Miettinen et al. (13) have reported that 60% patients with tumor diameter greater than 5 cm had poorer prognosis. H.Y also reported that prognostic factors of GIST include incidence site, onset age, tissue morphology, molecular genetics, immunohistochemical staining and tumor size, and tumor size was the most important risk factor for prognosis (37). In the present study, single factor analysis showed tumor diameters in adverse outcome patients were significantly higher than that in favorable outcome patients.
The incidence site of GIST now was also regarded as a prognostic factor in some studies. In the risk stratification of GIST, incidence site was a moderately important factor for assessment (3). However, in this study, we also did not see statistical difference on the risk classification in different parts of the gastrointestinal tract.
Although the main treatment of SIST is operation, it was reported that more than half of the patients had postoperative tumor recurrence and metastasis. GIST is not sensitive to traditional chemotherapy and radiotherapy, however, with the development of targeted therapy (tyrosine kinase inhibitor), treatment for GIST has been changing. IM is an inhibitor for tyrosine kinase receptors, it can selectively act on the c-kit tyrosine kinase receptor of GIST cells, so as to prevent the development of tumor. Yeh et al. (38) compared 22 moderate to high risk GIST patients who took IM 400 mg/day with 33 patients who did not take IM, and found the patients who took IM had significantly more overall survival time. In the present study, 14 patients used IM postoperatively, among which 2 patients had recurrence. One patient stopped IM after 2 months because of economic reason, and had metastasis to the liver after 8 months. After radiofrequency ablation treatment, this patient continued to take IM and the tumor disappeared after 6 months. The rest of the 49 cases did not take IM, 16 cases had postoperative adverse outcome, and all of them were moderate to high risk patients. The results of the present study showed that moderate to high risk patients who did not take IM had obviously more chances to have adverse outcome. During taking IM, doctors should pay attention to the monitoring of adverse drug reactions, and help patients to get a sustained, systemic treatment.
Studies showed GIST often recurrence within 1 year after surgery, or sometimes 10 years after surgery (39). Therefore, for SIST patients, doctors should pay attention to a long-term following-up after surgery.
The results of this research were mainly from the retrospective study, which had certain limitations: (I) a small number of patients; (II) all of the patients had different clinical manifestations such as gastrointestinal bleeding, abdominal mass, abdominal pain and discomfort such as anemia, there was no asymptomatic patients; (III) there was only 1 patient with tumor diameter less than 1 cm, which was not helpful for the study of small tumors (tumor diameter <1 cm).
Conclusions
SIST can happen at any age (mostly middle aged or older) and any part of the small intestine (mostly jejunum), according to tumor size and tumor location, SIST can appear a series of clinical manifestations (most common one gastrointestinal bleeding). With the development of various imaging techniques, the detection rate of SIST has been increased, small intestinal DSCT has the highest value in SIST detection and diagnosis, it can preliminarily identify malignant tumors from benign ones. However, the final diagnosis of SIST relies on pathological morphology and immunohistochemical staining, a higher expression of CD117 and DOG-1 can be sensitive markers for SIST or GIST. Tumor diameter 5.3 cm or higher and nuclear division number >5/50 can be independent risk factors to predict postoperative adverse outcome for SIST patients.
Acknowledgments
Funding: This study is funded by National Natural Science Foundation of China, No.81502009.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients gave informed consent to use their clinical data for research purposes. The hospital’s Protection of Human Subjects Committee approved the protocols used in this study, and the committee’s reference number is No. XJYYLL-2015270.
References
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [Crossref] [PubMed]
- Cao H, Zhang Y, Wang M, et al. Prognostic analysis of patients with gastrointestinal stromal tumors: a single unit experience with surgical treatment of primary disease. Chin Med J (Engl) 2010;123:131-6. [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10:1045-52. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003;226:527-32. [Crossref] [PubMed]
- Grover S, Ashley SW, Raut CP. Small intestine gastrointestinal stromal tumors. Curr Opin Gastroenterol 2012;28:113-23. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Bülbül Doğusoy G. Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol 2012;23:203-11. [Crossref] [PubMed]
- Bhalgami R, Manish K, Patil P, et al. Clinicopathological study of 113 gastrointestinal stromal tumors. Indian J Gastroenterol 2013;32:22-7. [Crossref] [PubMed]
- Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155-66. [Crossref] [PubMed]
- Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-89. [Crossref] [PubMed]
- ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii49-55. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Guller U, Tarantino I, Cerny T, et al. Revisiting a dogma: similar survival of patients with small bowel and gastric GIST. A population-based propensity score SEER analysis. Gastric Cancer 2017;20:49-60. [Crossref] [PubMed]
- Saito T, Ueno M, Ota Y, et al. Histopathological and clinical characteristics of duodenal gastrointestinal stromal tumors as predictors of malignancy. World J Surg Oncol 2013;11:202. [Crossref] [PubMed]
- Han IW, Jang JY, Lee KB, et al. Clinicopathologic analysis of gastrointestinal stromal tumors in duodenum and small intestine. World J Surg 2015;39:1026-33. [Crossref] [PubMed]
- Judson I, Bulusu R, Seddon B, et al. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res 2017;7:6. [Crossref] [PubMed]
- Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898-905. [Crossref] [PubMed]
- Zang L, Hu WG, Yan XW, et al. Laparoscopic treatment for small intestinal bleeding: a report of 77 cases. J Laparoendosc Adv Surg Tech A 2010;20:521-5. [Crossref] [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Constantin VD, Socea B, Popa F, et al. A histopathological and immunohistochemical approach of surgical emergencies of GIST. An interdisciplinary study. Rom J Morphol Embryol 2014;55:619-27. [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Gutierrez JC, De Oliveira LO, Perez EA, et al. Optimizing diagnosis, staging, and management of gastrointestinal stromal tumors. J Am Coll Surg 2007;205:479-91 (Quiz 524).
- Valsangkar N, Sehdev A, Misra S, et al. Current management of gastrointestinal stromal tumors: Surgery, current biomarkers, mutations, and therapy. Surgery 2015;158:1149-64. [Crossref] [PubMed]
- Teoh WC, Teo SY, Ong CL. Gastrointestinal stromal tumors presenting as gynecological masses: usefulness of multidetector computed tomography. Ultrasound Obstet Gynecol 2011;37:107-9. [Crossref] [PubMed]
- Hirasaki S, Fujita K, Matsubara M, et al. A ruptured large extraluminal ileal gastrointestinal stromal tumor causing hemoperitoneum. World J Gastroenterol 2008;14:2928-31. [Crossref] [PubMed]
- Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS 2006;114:192-200. [Crossref] [PubMed]
- Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Kara T, Serinsoz E, Arpaci RB, et al. Contribution of DOG1 expression to the diagnosis of gastrointestinal stromal tumors. Pathol Res Pract 2013;209:413-7. [Crossref] [PubMed]
- Sözütek D, Yanik S, Akkoca AN, et al. Diagnostic and prognostic roles of DOG1 and Ki-67, in GIST patients with localized or advanced/metastatic disease. Int J Clin Exp Med 2014;7:1914-22. [PubMed]
- Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Tabrizian P, Sweeney RE, Uhr JH, et al. Laparoscopic resection of gastric and small bowel gastrointestinal stromal tumors: 10-year experience at a single center. J Am Coll Surg 2014;218:367-73. [Crossref] [PubMed]
- Oida Y, Motojuku M, Morikawa G, et al. Laparoscopic-assisted resection of gastrointestinal stromal tumor in small intestine. Hepatogastroenterology 2008;55:146-9. [PubMed]
- Cai W, Wang ZT, Wu L, et al. Laparoscopically assisted resections of small bowel stromal tumors are safe and effective. J Dig Dis 2011;12:443-7. [Crossref] [PubMed]
- Yan H, Marchettini P, Acherman YI, et al. Prognostic assessment of gastrointestinal stromal tumor. Am J Clin Oncol 2003;26:221-8. [Crossref] [PubMed]
- Yeh CN, Chen TW, Wu TJ, et al. Treatment of patients with advanced gastrointestinal stromal tumor of small bowel: implications of imatinib mesylate. World J Gastroenterol 2006;12:3760-5. [Crossref] [PubMed]
- García de Polavieja Carrasco M, de Juan Ferré A, Mayorga Fernández M. Gastrointestinal stromal tumours at present: an approach to burning questions. Clin Transl Oncol 2010;12:100-12. [Crossref] [PubMed]
Cite this article as: Zhao L, Zhao Z, Wang W, Zhao W, Tuo S, Shi Y, Zhang W, Chen L, Hong L, Yang J, Lu W, Wu Q, Wang J, Wu K. Current characteristics on small intestinal stromal tumor—a case control study. Ann Palliat Med 2020;9: