
Effect of intravitreal ranibizumab pretreatment on vitrectomy in young patients with proliferative diabetic retinopathy
Introduction
The epidemic of diabetes mellitus (DM) has been escalating worldwide in recent years and are becoming a global burden of public health (1-3). It is estimated that DM population is expected to rise to 642 million by 2040 (1). More strikingly, several studies have showed that the onset age of DM is becoming earlier (4-6), even in the type2 DM, which was previously considered to occur in older adults. Development and progression of proliferative diabetic retinopathy (PDR) is much quicker in young diabetic patients than in older counterpart, which is the leading cause of vision impairment in diabetic patients. A higher risk of blindness was noted in younger patients with PDR than in older patients (7-13).
Pars plana vitrectomy (PPV) is the mainstay treatment for PDR patients, which involves the removal of fibrovascular membranes and the release of vitreoretinal traction (14). However, vitrectomy for PDR in young patients may be challenging due to the broad vitreoretinal adhesion and severe active fibrovascular proliferation. Moreover, Younger patients who received vitrectomy for PDR have a higher rate of postoperative complications, like recurrent vitreous hemorrhage (VH), recurrent detachment, and neovascular glaucoma (NVG) (15,16). Therefore, younger patients with PDR need our special attention.
Several studies have shown that VEGF is a pivotal causative factor in eye diseases that are characterized by neovascularization or increased vascular permeability, such as PDR (17,18). Intravitreal injection of anti-VEGF agents before vitrectomy has been widely used as an adjunctive therapy in PDR patients (19-24). Among them, young PDR patients should be one of the best indications for anti-VEGF treatment prior to vitrectomy since younger patients usually have more active fibrovascular proliferation and more intraoperative and postoperative bleeding. However, there were few studies that investigated on the effect of anti-VEGF pretreatment for vitrectomy in young PDR patients (15). Therefore, this study was undertaken to focus on the effects of ranibizumab (Lucentis; Novartis Pharma Schweiz AG Inc., Schaffhauserstrasse 4332 Stein, Switzerland), one of the VEGF inhibitors, on vitrectomy surgery in young patients with PDR.
Methods
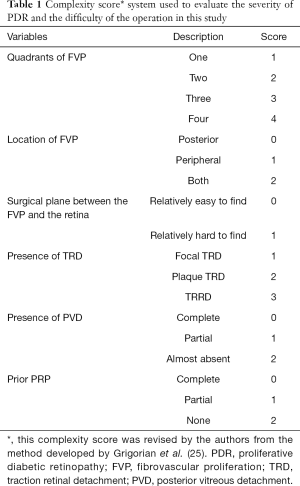
This is a prospective, non-randomized, comparative study. From October 2016 to June 2017, consecutive patients (<40 years old) undergoing PPV for PDR were recruited for the study in the ophthalmology department of Peking University Third Hospital. Exclusion criteria included: (I) history of vitrectomy in the study eye, (II) history of receiving anti-VEGF injection in the recent three months, (III) history of other ischemic or inflammatory intraocular disorders, (IV) history of thromboembolic events (including myocardial infarction or cerebral vascular events), (V) known coagulation abnormalities or current use of anticoagulative medication. The off-label use of intravitreal ranibizumab (IVR) on PDR and the potential risks (in particular the potential for thromboembolic events, endophthalmitis and uveitis) and benefits of its use were discussed at length with all patients at the time of recruitment. Patients who accepted IVR prior to PPV were included in the IVR group, and patients who declined IVR prior to PPV were included in the control group. A complexity score was used to quantify the severity of PDR and to match the two groups. The assigning of complexity scores (Table 1) was revised by the authors from the method developed by Grigorian et al. (25). The hospital ethics committee granted approval for the project, and written informed consent was obtained from all study participants in accordance with the tenets of the Declaration of Helsinki for research involving human subjects.
Full table
All patients received a detailed ophthalmologic examination to establish a baseline and were re-evaluated after surgery. Preoperative, intraoperative, and postoperative data were collected for each patient. Baseline data included demographics including age and gender; systemic factors including type of diabetes, history of hypertension, hemoglobin A1c levels before the surgery, and renal function; and ophthalmic factors including preoperative best corrected visual acuity (BCVA), previous history of pan-retinal photocoagulation (PRP), lens status, the presence of preoperative iris neovascularization, and complexity score. BCVA was measured using a decimal visual acuity chart, and the decimal visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR) equivalent for statistical analysis.
In the IVR group, ranibizumab (0.5 mg/0.05 mL) was injected 3-5 days prior to PPV. All patients in this study underwent a standard 23-gauge three-port vitrectomy that was performed with a CONSTELLATION vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA). The maximum speed of the vitreous cutter was 7,500 cpm. Preretinal membranes were dissected or peeled using segmentation and/or delamination techniques. Bimanual dissection was not used in all patients. The intraoperative clinical evaluation was based on the following surgical parameters: intraoperative bleeding during the dissection of fibrovascular tissue, the use of endodiathermy, the need for relaxing retinotomy, the use of PFCL, the formation of iatrogenic retinal tears, the need for silicone oil endotamponade, and vitrectomy time. Intraoperative bleeding was divided into mild and severe and was defined as mild if it was stopped by increasing the infusion pressure or gently pressing with a blunt instrument and severe if endodiathermy was required. An evaluation form was completed by the surgeon immediately after the surgery. Moreover, a second vitreoretinal specialist who was blinded to grouping information observed the operation on the video system and reconfirmed the evaluation form. The postoperative clinical evaluation included the incidence of early (≤4 weeks) and late (>4 weeks) postvitrectomy hemorrhage, postoperative NVG, recurrent detachment, and BCVA at 1 month and 1 year of follow up. In eyes without gas/silicone oil tamponade, postoperative hemorrhage in the vitreous cavity was graded according to the scale defined by the Diabetic Retinopathy Vitrectomy Study Group [2005], as follows: mild, with visible fundus details; greater than mild, with no visible fundus details, with or without an orange fundus reflex. In eyes with gas/silicone oil tamponade, postoperative preretinal blood was classified as: mild, with blood clots that have a total area of ≤3 disk area; or greater than mild, with blood clots that have a total area of >3 disk area. Data for ocular and systemic adverse events after IVR were also collected.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation, and independent t-tests were performed to make comparisons between the two groups. To examine the percentage differences between the two groups, statistical analysis of the data was performed with a Chi-squared test or Fisher’s exact test. Spearman’s nonparametric correlation analysis was used to evaluate the association between the scale of the complexity score and the occurrence of iatrogenic retinal breaks and the use of PFCL and silicone oil. A value of P<0.05 was considered to denote statistical significance. The statistical analysis of the data was performed by using SPSS V20.0 software for Windows (SPSS, Chicago, Illinois, USA).
Results
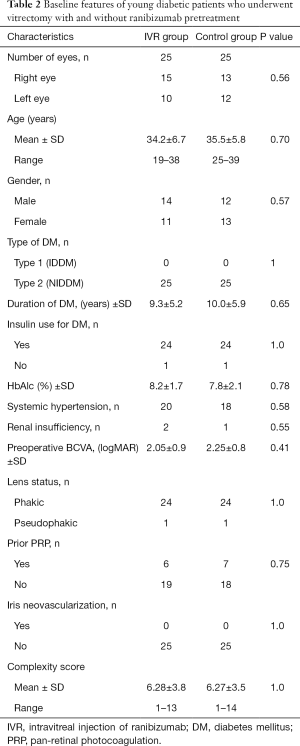
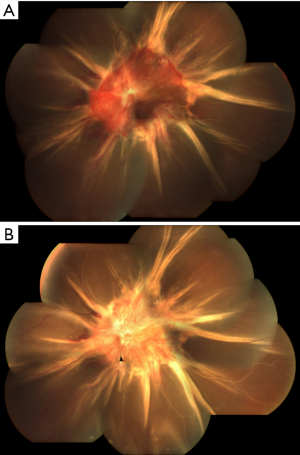
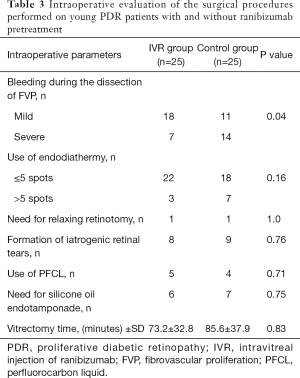
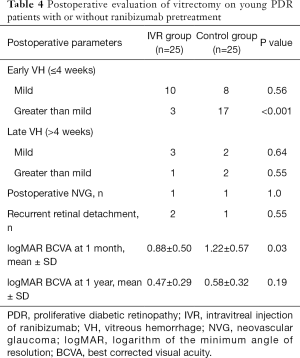
From October 2016 to June 2017, 64 consecutive patients (<40 years old) undergoing PPV for PDR were recruited for this prospective study. After they were matched by complexity score, 25 eyes in each group were enrolled. Baseline characteristics were well balanced in both groups and are summarized in Table 2. Clinical examination showed significantly vascularized preretinal membranes in young diabetic patients (Figure 1A). In the IVR group, the interval between IVR and PPV was 3.8±0.8 days. Marked attenuation of the vasculature was observed after ranibizumab injection (Figure 1B). No IVR-related ocular or systemic complications were observed in the IVR group. None of the patients demonstrated the occurrence or progression of TRD after the administration of ranibizumab. The intraoperative clinical evaluations of the operated eyes are presented in Table 3. Intraoperative bleeding was significantly reduced in the IVR group compared to the control group (P=0.04). Accordingly, endodiathermy was used less in the IVR group, and vitrectomy time was 73.2±32.8 minutes in the IVR group and 85.6±37.9 minutes in the control group (P=0.83). However, the need for relaxing retinotomy, the use of PFL, the formation of iatrogenic retinal tears, and the need for silicone oil endotamponade were not affected by IVR pretreatment. In both the IVR group and the control group, there was a significant correlation between the complexity score and the use of PFL, the formation of iatrogenic retinal tears, and the need for silicone oil endotamponade (Spearman, r=0.65, 0.81, and 1.0, respectively, P<0.001 in the IVR group; and r=0.52, 0.55, and 0.90, respectively, P<0.01 in the control group). Postoperative clinical evaluations of the operated eyes are presented in Table 4. Early (≤4 weeks) postvitrectomy hemorrhage (greater than mild) was significantly reduced in the IVR group than in the control group (P<0.001). logMAR BCVA was better in the IVR group than in the control group 1 month after vitrectomy (P=0.03), whereas logMAR BCVA at 1 year was similar between the two groups (P=0.19). There were no significant differences in late (>4 weeks) postvitrectomy hemorrhage, postoperative NVG, recurrent detachment between the two groups.
Full table
Full table
Full table
Discussion
Younger patients who underwent vitrectomy for PDR display more aggressive nature distinguished from the older patients (15,16). First, younger patients with PDR commonly have broad and active fibrovascular proliferation, and vigorous growth of new vessels in the membrane can cause massive bleeding during surgery. Second, in younger patients, the posterior hyaloid is almost completely attached to the retina, which makes accessing the subhyaloid space difficult. Creation of posterior vitreous detachment may induce iatrogenic retinal break and bleeding during surgery. Third, it is more common to see the dispersion of residual blood and rebleeding from clot lysis during the early days following postvitrectomy in young diabetic patients. Finally, younger patients are at greater risk of having recurrent VH and postoperative NVG after vitrectomy. Therefore, the general prognosis for younger diabetic patients is much poorer than that for the older population. In this study, young patients who receive PPV for PDR was defined as patients less than 40 years old, because patients with early-onset DM usually start before 30 years of age, and there may be a five- to ten-year lag between disease onset and surgical intervention.
Preoperative anti-VEGF therapy has been widely used as an adjunct for PDR surgery (19-24). However, the effect of anti-VEGF administration in young diabetics has rarely been evaluated independently in previous studies. In the current study, we focused our investigation on young diabetics and evaluated the effect of IVR pretreatment on this specific population. The current study shows that vigorous growth of new vascular component in the proliferative membrane in young PDR patients (Figure 1A). in the IVR group, in order to avoid exacerbation of fibrosis and traction retinal detachment, IVR was performed 3–5 days prior to PPV. Marked attenuation of new vessels was observed after ranibizumab injection (Figure 1B). The regression of neovascularization contributed to a technically easier surgical procedure. The severity of intraoperative bleeding was significantly reduced in the IVR group than in the control group. Accordingly, the use of endodiathermy and total surgical time were decreased after ranibizumab pretreatment. These findings are in accordance with other studies in the literature. Previous publication also showed that anti-VEGF pretreatment in PDR patients can reduce the incidence of iatrogenic retinal breaks and the use of silicone oil tamponade during the surgery (24). However, this was not proved in our study. In the current study, we used the complexity score to quantify the severity of PDR. The complexity score system used in this study (Table 1) was revised by the authors from the method developed by Grigorian et al. (25). The revised complexity score is more comprehensive than the previous scheme and may more accurately reflect the severity of the vitreoretinal condition in surgical cases. In this study, we found that IVR pretreatment did not reduce the rate of relaxing retinotomies, the risk of iatrogenic retinal breaks, and the use of perfluorocarbon liquid (PFCL) or silicone oil tamponade in young PDR patients. Instead, the complexity score was strongly correlated with the risk of iatrogenic retinal breaks and the use of PFCL and silicone oil tamponade. Our findings suggest that in young PDR patients, the difficulty of the surgery and the incidence of intraoperative complications depends mainly on the complexity of the surgical case, in terms of the extension of fibrovascular proliferation and adhesion to the retina, rather than bleeding itself.
Despite advancements in surgical technique, postvitrectomy VH is frequently observed in diabetic patients (20,22,26), especially in the young PDR patients. It can have an early or a late onset. Early postoperative VH is more likely caused by release of blood from the peripheral vitreous skirt, rebleeding from vascular membranes transected at surgery, and persistent vitreoretinal traction, whereas the recurrent VH in the late postoperative period is likely to be caused by neovascularization growth at the sclerotomy sites or retinal periphery, and in a high proportion of rebleed cases, the source of hemorrhage is not well identified (20,22,27). Postvitrectomy hemorrhage might clear spontaneously, but sometimes it requires additional surgical procedures. Reproliferation after surgery is likely to occur in the presence of significant preretinal bleeding in the eyes when silicone oil infusion is used, that can cause recurrent retinal detachment. Moreover, younger age is a significant risk factors for postoperative NVG and the presence of NVG was associated with worse outcome in the younger group (15,16). Whether these postoperative complications could be prevented in young PDR patients by IVR pretreatment was also investigated in this study. In this study, we found the occurrence of early postvitrectomy hemorrhage was significantly reduced in the IVR group compared to the control group. Accordingly, visual rehabilitation was faster in the IVR group than in the control group. However, there were no marked differences in late onset VH, recurrent retinal detachment and postoperative NVG between the two groups. Furthermore, the final visual recovery at one year after surgery were also similar for both groups. These findings suggest in young PDR patients IVR pretreatment can prevent early rather than late postoperative complications, which accelerates the visual recovery in the early postoperative period but has little effect on final visual outcome.
There were some limitations to the current study. First, this was a non-randomized study with relatively small number of patients. Hence, although a complexity score was used to quantify the severity of PDR and match the two groups, it was unavoidable that there would be some selection bias in the study. Second, the surgeons were not masked to the grouping information. This would also cause some bias in the decision making by surgeons during surgery. Third, this study was carried out in a single center, and the surgeries were not performed by a single operator. This was also a potential source of bias. Nevertheless, our study was the first prospective comparative study to evaluate the effect of IVR pretreatment on vitrectomy in young PDR patients (<40 years old). We found IVR pretreatment is a safe and effective adjunct to vitrectomy to reduce intraoperative and early postvitrectomy bleeding thus should be suggested in young PDR patients. However, IVR does not reduce the incidence of intraoperative and late postoperative complications in young PDR patients. The risk of iatrogenic retinal breaks and silicone oil use are closely correlated with the complexity score of the surgical cases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional ethics committee of Peking University Third Hospital (No. 2015092). The research followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
References
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88-98. [Crossref] [PubMed]
- Pandey A, Chawla S, Guchhait P. Type-2 diabetes: Current understanding and future perspectives. IUBMB Life 2015;67:506-13. [Crossref] [PubMed]
- Nanditha A, Ma RC, Ramachandran A, et al. Diabetes in Asia and the Pacific: Implications for the Global Epidemic. Diabetes Care 2016;39:472-85. [Crossref] [PubMed]
- Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664-72. [Crossref] [PubMed]
- Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med 2015;32:1119-20. [Crossref] [PubMed]
- Pan J, Jia W. Early-onset diabetes: an epidemic in China. Front Med 2018;12:624-33. [Crossref] [PubMed]
- Singerman LJ, Weaver DT. PDR in juvenile onset diabetics: high-risk proliferative diabetic retinopathy in juvenile onset diabetics. Retina 1981;1:18-26. [PubMed]
- Wong J, Molyneaux L, Constantino M, et al. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008;31:1985-90. [Crossref] [PubMed]
- Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520-6. [Crossref] [PubMed]
- Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527-32. [Crossref] [PubMed]
- Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107:237-43. [Crossref] [PubMed]
- Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989;107:244-9. [Crossref] [PubMed]
- Zou W, Ni L, Lu Q, et al. Diabetes Onset at 31-45 Years of Age is Associated with an Increased Risk of Diabetic Retinopathy in Type 2 Diabetes. Sci Rep 2016;6:38113. [Crossref] [PubMed]
- El Rami H, Barham R, Sun JK, et al. Evidence-Based Treatment of Diabetic Retinopathy. Semin Ophthalmol 2017;32:67-74. [Crossref] [PubMed]
- Huang CH, Hsieh YT, Yang CM. Vitrectomy for complications of proliferative diabetic retinopathy in young adults: clinical features and surgical outcomes. Graefes Arch Clin Exp Ophthalmol 2017;255:863-71. [Crossref] [PubMed]
- Goto A, Inatani M, Inoue T, et al. Frequency and risk factors for neovascular glaucoma after vitrectomy in eyes with proliferative diabetic retinopathy. J Glaucoma 2013;22:572-76. [Crossref] [PubMed]
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480-7. [Crossref] [PubMed]
- Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol 2002;133:373-85. [Crossref] [PubMed]
- Rizzo S, Genovesi-Ebert F, Di Bartolo E, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 2008;246:837-42. [Crossref] [PubMed]
- Ahmadieh H, Shoeibi N, Entezari M, et al. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009;116:1943-8. [Crossref] [PubMed]
- Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009;116:927-38. [Crossref] [PubMed]
- Ahn J, Woo SJ, Chung H, et al. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011;118:2218-26. [Crossref] [PubMed]
- Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol 2013;156:106-15.e2. [Crossref] [PubMed]
- Zhao XY, Xia S, Chen YX. Antivascular endothelial growth factor agents pretreatment before vitrectomy for complicated proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Br J Ophthalmol 2018;102:1077-85. [PubMed]
- Grigorian RA, Castellarin A, Fegan R, et al. Epiretinal membrane removal in diabetic eyes: comparison of viscodissection with conventional methods of membrane peeling. Br J Ophthalmol 2003;87:737-41. [Crossref] [PubMed]
- Yau GL, Silva PS, Arrigg PG, et al. Postoperative Complications of Pars Plana Vitrectomy for Diabetic Retinal Disease. Semin Ophthalmol 2018;33:126-33. [Crossref] [PubMed]
- Novak MA, Rice TA, Michels RG, et al. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology 1984;91:1485-9. [Crossref] [PubMed]


