A survey of clinical and laboratory characteristics of dengue fever epidemic from 2014 to 2018 in Guangzhou, China
Introduction
Dengue, mosquito-borne disease and primarily transmitted to humans by the female Aedes mosquitos (1), is the second-most globally prevalent vector-borne disease in the world, only behind malaria in terms of morbidity and mortality (2). It is estimated that dengue fever (DF) incidence has increased 30-fold over the last 50 years (3). The rapid increase of DF incidence has become severe public health, threating approximately half of the world’s population, especially in Southeast Asia, the west Pacific Ocean regions, and southern Africa, which due to geographical environment, population density, and other factors (4). DENV is a member of the genus Flavivirus, family Flaviviridae, positive-strand RNA viruses, which is a single-stranded, positive-sense, RNA virus with a genome of about 11 kb (5,6). The causative agents of dengue disease are antigenically divided into four distinct serotypes, DENV1 through DENV4 (7). Infection with DENV results in varying degrees of pathological conditions, ranging from mild asymptomatic DF to severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), which may turn fatal (8). The first exposure of an individual to any of the four dengue virus serotypes is known as primary dengue infection, with high titers of immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies appearing in 3–5 and 6–10 days respectively, after the onset of infection (1). A secondary infection, with a previously unencountered DENV serotype, usually results in classical DF, while 23% of cases develop into DHF or progress to DSS and death (1).
A key role in dengue therapy and control of the dengue epidemic is an early diagnosis of DF. Until now, the routine diagnostic methods to confirm DF include a combination of clinical dengue symptoms, viral isolation, viral RNA detection, and serological assays (9). However, none of these methods are sufficiently sensitive and specific to be used as a stand-alone diagnostic tool (10). Although Virus isolation was the “gold standard” for the diagnosis of DF, it was time-consuming, required cell facilities, and yielded low detection rates (11). RNA and other molecular tests have been widely applied for the rapid detection and identification of dengue virus serotypes in clinical diagnosis. However, in many poorly equipped laboratories of China, molecular diagnostics is still not possible. Other serological assays, including detection of IgM/IgG, are the most commonly used methods because of smooth operation, inexpensive, and saving time, with poor sensitivity and specificity. Nonstructural protein 1 (NS1) is a glycoprotein secreted by DENV infected mammalian cells and is essential for viral replication and viability (12). It has been found that the utility of the NS-1 antigen has underlined its importance in the early stages of DENV infection because the NS1 antigen is detectable in blood from the first day after the onset of fever up to Day 9 (13), is found earlier than IgM (14). So NS1 tests enzyme-linked immunosorbent assay (ELISA) may be a better choice for DENV detection (15).
In mainland China, DF is a severe infectious disease by the Ministry of Health of China, and sporadic dengue cases have been reported in Guangdong, Zhejiang, and Fujian, which are in Southeast China (16). Guangzhou, the capital of Guangdong Province, with the highest population density of more than 1,800 persons per square kilometer, is a typical city that has experienced annual DENV transmission, accounting for more than 50% of the DENV cases in China (6,17). In July 2014, one of the most significant outbreaks of dengue occurred in Guangzhou since 1986, providing great concern of the society. The reasons for the dengue epidemic are complex. In the study, we will investigate the probable origin of dengue in Guangzhou, China.
Methods
Clinical information included age, gender, Fever days before treatment, other epidemiological characteristics, and samples were collected from patients at Nanfang Hospital, Southern Medical University, from 2014 to 2018 in Guangzhou, China. Full blood counts were completed by automatic blood cell analyzer (SYSMEX XE-5000) for all patients. Detection Kit analyzed laboratory diagnostic tests including DENV-RNA, DENV-IgM/IgG, NS1 antigen detection for Dengue virus RNA (RT-PCR-Fluorescence Probing, Hua yin Medical Technology Co Ltd, Guangzhou, China), Diagnostic Kit for Dengue IgG/IgM Antibody (Colloidal Gold, Wondfo Biotech, Guangzhou, China) and Diagnostic Kit for Dengue VirusNS1 (ELISA, WANTAI BioPharm, Beijing, China). Laboratory Biochemical tests were analyzed by Cobas 8000 (Roche Diagnostics GmbH).
Results
Description of the outbreak
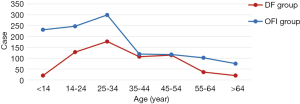
A total number of 1,792 of patients were included in our study, from which 602 cases were classified as DF group and 1,190 cases as other febrile illnesses (OFI) group. In the DF group, it consisted of 51.8% male patients which gender ratio was 1.08:1 (male/female, P>0.05), with the median age, was 32 years old (range, 1 day to 81 years). In the OFI group, it consisted 59.2% of male patients in which gender ratio was 1.45:1 (male/female, P<0.05), with the median age 27 years old (range, 5 months to 89 years). 87.38% of patients were in the 14–54 years age group of DF group, the main peak appeared in the 25–34 years age group (29.40%). 65.3% of patients were in 0–34 age group of OFI group, with three main peak: 0–14 years old (19.41%), 15–24 years old (20.76%) and 25–34 years old (25.13%) (Table 1, Figure 1).

Full table
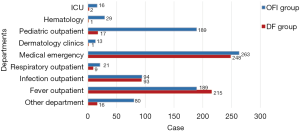
Cases from different departments
In the DF group, patients were from fever outpatient 215 cases (35.7%) and medical emergency 248 cases (41.2%). In the OFI group, suffers were from fever outpatient 189 cases (21.1%), medical emergency 263 cases (29.4%), and 189 cases of pediatric outpatient (21.1%, P<0.001), shown in Figure 2.
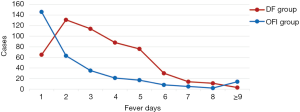
Fever days before treatment
The distribution of fever days before treatment was shown in Figure 3, mainly focused within 5 days of fever days, which accounted for 89.10% in the DF group and accounted for 91.70% in the OFI group. In the DF group, the main peak appeared in the 2 fever days before treatment (24.60%), and in the OFI group, patients with the main peak in 1 fever day before treatment (46.90%).
Clinical symptoms
The clinical symptoms in both of DF group and OFI group mainly were typical or mild manifestations with upper respiratory tract infection, as pharyngeal hyperemia and adenoids. The primary symptoms of the DF group were presented with were fever (100%), myalgia (34.77%), Pharyngeal hyperemia (31.33%), headache (25.65%), adenoids (19.62%), and rash (13.25%). The results of clinical symptoms were presented in Table 2, and the clinical symptoms of myalgia, headache, rash, weak, chills, follicular hyperplasia between the DF group, and OFI group were with P value less than 0.001. In the DF group, myalgia was the most common clinical symptom, accounting for 34.77%, with 7.14% in the OFI group. In the OFI group, Pharyngeal hyperemia was the most common clinical symptom, accounting for 27.24%, and the next symptom was adenoids (21.26%) (Table 2).
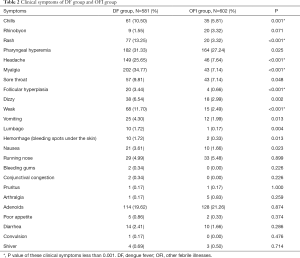
Full table
Laboratory diagnostic tests
In the experiment, 42 paired serum samples were collected to detect both DF-NS1 and RNA, and 356 paired serum samples were collected to detect both DF-NS1 and DENV-IgM/IgG during the acute stage of illness. Among the 39 cases with DF-NS1 positive, DF-RNA, with 24 positive cases and 15 negative cases. The other 4 cases detected by DF-NS1 and DF-RNV were both negative. The sensitivity and specificity of DF-RNA were 61.54% and 100%, respectively, compared to the DF-NS1.
DENV-IgM in both groups was statistically significant, with DENV-IgM in the DF group were stronger (Z=−7.863, P<0.001), and DENV-IgG were no statistically significant (Z=−1.212, P=0.226). Among the 284 DF-NS1 positive cases, IgM antibodies were detected from 162 cases, and IgG antibodies were detected from 24 cases. Among 72 cases with DF-NS1 antigen-negative, IgM and IgG antibodies were detected from 3 cases, which had lower sensitivity (IgM 57.04%, IgG 8.45%) and high rate of omission diagnostic (IgM 42.96%, IgG 91.55%), compared to the DF-NS1 (Tables 3-5).

Full table

Full table

Full table
Laboratory biochemical tests
In DF group, 39 (37.14%) of 105 cases had elevated ALT levels, 83 (76.85%) of 108 cases had elevated AST levels, 17 (32.08%) of 53 cases had elevated CK levels and 32 (42.67%) of 75 cases had elevated CRP levels, compared with 5 (13.51%) of 37 cases had elevated ALT levels, 19 (30.65%) of 62 had elevated AST levels, 2 (6.06%) of 33 cases had elevated CK levels and 43 (69.35%) of 62 cases had elevated CRP levels of the OFI patients. AST, ALT, CK, and CRP levels were significantly higher in the DF group than the OFI patients (P<0.01). In both groups, no significant difference in other cardiac function tests, such as LDH, α-HBDH, CK-MB, and other biochemical tests, including TP, ALB, PCT. In the DF group, AST levels are higher than ALT levels (Table 6).
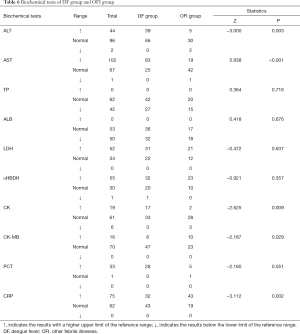
Full table
Full blood count
The prominent manifestations were thrombocytopenia (occurring in 28.07% of the DF group, compared to 5.18% of OFI group) and leucopenia (occurring in 43.27% of the DF group and 3.63% of OFI group). From the data in our stud, 44.44% had decreased LYM%, 30.21% had elevated NEU% levels, 54.58% had elevated MONO%, and 7.41%had decreased Hgb had decreased PLT in DF group, compared with 54.40% decreased LYM%, 45.60% elevated NEU%, 17.10% elevated MONO%, and 19.17% decreased Hgb in OFI group. In all indicators of full blood count, WBC, LYM%, NEU%, MONO%, Hgb, PLT were statistically significant between the DF group and OFI group (P<0.001) (Table 7).
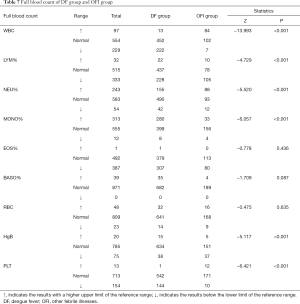
Full table
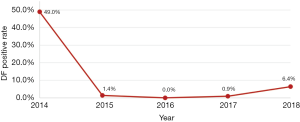
Annual incidence
From October to December 2014, DF incidence of all fever cases was 49.0% (584/1,193), compared with 1.4% (3/219) in 2015, 0% (0/50) in 2016, 0.9% (1/110) in 2017 and 6.4% (14/220) in 2018 (P<0.001) (Figure 4).
Discussion
During 2014, the largest dengue epidemic occurred in Guangdong province in the past 25 years, China (18). Especially in Guangzhou, accounting for 90% of all of the reported dengue cases occurred in this outbreak (statistics from Guangdong province Health and Family Planning Commission) (17).
On the one hand, the causes of the massive dengue outbreak were unknown, but there are some factors associated with it, such as climate change, globalization, travel, and trade (19). Firstly, Guangzhou is the most densely populated city in southern China, surrounded by many dengue-endemic countries. It has a humid subtropical climate influenced by the Asian monsoon (20). Except for the influence of temperature, which was be found positively associated with dengue incidence, there are other atmospheric factors, such as relative humidity and wind velocity may also increase the risk of DF infection (21). Secondly, Guangzhou is one of the most important international financial centers in Southeast Asia because of globalization. Increasingly overseas commercial investors, business people, laborers, and foreign tourists have led to more international exchanges in this region, which could increase the risk of imported DF cases from endemic areas (6,22). It was reported that the main reasons for the pattern of the serve outbreak in 2014 were related to the date of the first imported case, unusually high precipitation in 2014, interventions, and vertical transmission (23). Thirdly, Guangzhou has a current population of more than 10 million, which has a vast mobile population city in China. The migration increased in the susceptible population promotes the transmission of infectious diseases and creates significant challenges to prevent and control dengue endemic (2,24). Finally, the rapid development of urbanization and construction in this region may have also contributed to the transmission of dengue. On the other hand, human factors contribute to the risks of morbidity and the spread of dengue, including both urban and rural settlement patterns (20). Recent data suggests that dengue vaccines are at a crossroads even with modest efficacy (25,26), so the only effective way to prevent DF is controlling mosquito vector.
This study showed that DF could occur in any age and any sexes. Males were found to be affected by DF slightly more than females in our study, but this difference was not statistically significant (P>0.05). Initially, dengue infection was thought to be a disease of children, but it has been reported that the elderly had a comparatively higher incidence of acquiring dengue infection (27). But in this study, adults aged 25–34 (29.40%) years with the main peak, and youngster aged 14–24 (21.26%) with a secondary peak while the elderly aged 45–54 (18.94%) with a third peak appeared in DF group; This finding is in general agreement with other studies (17,28,29). It may be the youngsters and adults have a larger crowd size, like to spend more time outdoors sports and ignore the protection of mosquito biting. And the aged 45–54 years like planting flowers in household courtyards, which provides many containers with water around the house, helping mosquito breeding (22). All of these factors may result in the middle-aged and elderly more susceptible to dengue infection. It was reported that the elderly infected with the dengue virus appears to be more likely to develop severe illness (30). In the OFI group, adult aged 25–34 (25.13%) years are the highest incidence, and the aged 14–24 (20.76%) years with a secondary peak while child aged less than 14 (19.41%) years with a third peak, who are prone to upper respiratory tract infection because of low immunity, while adults will pay more attention to the prevention of child mosquito bites which may reduce the incidence of DF infection.
From the distribution of specimens for inspection departments, patients are from Fever outpatient, and Medical emergency indicated that the symptoms in the DF group and the OFI group are characterized by acute onset. In the OFI group, the proportion of pediatric outpatients 189 cases (21.10%) was significantly higher than the DF group, which guided clinical diagnosis: when the child has a fever may be a non-dengue infection. According to our data statistics, we found that approximately 90% of all fever patients had gone to the hospital within 5 fever days before treatment, but in the OFI group fever days before treatment was significantly earlier than the DF group, with 46.90% patients mainly focused within 1 day of fever days. The patients of the OFI group were primarily for children whose parents paid more attention to their illness, for they usually with poor tolerance to fever.
The clinical symptoms in all fever patients mainly were typical or mild manifestations with upper respiratory tract infection, as pharyngeal hyperemia and adenoids. However, the clinical symptoms, including myalgia, headache, rash, weak, chills, follicular hyperplasia, were with P<0.001 between the DF group and OFI group. It was thought that myalgia could be a specific sign of clinical symptoms for DF, which was the most common clinical symptom in the DF group.
In our study, we used three methods for the diagnosis of DF, including DF-RNA, DENV-IgM, and IgG, DF-NS1. The test of DENV-IgM and IgG was the most rapid and convenient serological technique, which had lower sensitivity (IgM 57.04%, IgG 8.45%) and a high rate of omission diagnostic (IgM 42.96%, IgG 91.55%), compared to the DF-NS1 test. Therefore, only the detection of IgM and IgG antibodies may miss a lot of DF diagnoses. IgM and IgG antibodies persisted in the serum for more than 3 months, which could be a limiting factor in confirmatory diagnosis. The detection of DENV-IgG was not considered authentic, which cross-reactivity with other closely related members of flaviviruses (9). Some studies founded that DENV-IgM lever increased highly in primary infections 3–5 days after the onset of fever, which levels peaked in the serum about two weeks after the onset of symptoms, and DENV-IgG increased 1–2 days rapidly after the onset of fever (31). It was thought that DENV-IgM tests were more suitable for early diagnosis of DF, and the level of DENV-IgG was used to find a secondary infection by IgG needing a >fourfold rise in titer in paired acute and convalescent sera. The sensitivity and specificity of DF-RNA were 61.54% and 100%, respectively, compared to the DF-NS1 test. In all specimens, 38.5% cases (15/39) were DF-NS1 positive but negative for DF-RNA, and this rate was like a previous report (37%) (32). It may be half-life period of NS1 protein was longer; the NS1 circulating in a patient’s blood is longer periods than viral RNA (33). So NS1 antigen remained positive which affords a valuable diagnostic test after DENV-RNA amplicon disappeared (34). Besides, there may be false-positive results of the detection of DF-NS1. Compared to virus isolation and RT-PCR methods, they offer a cheaper and more convenient option that only requires basic technical training (35). Particularly during the early stage of infection before the induction of a humoral immune response to the virus (34), NS1 antigen immunoassays is a rapid diagnostic test and is also effective and its ability to detect the dengue virus infection early in the course of disease helps in opting for appropriate treatment and management planning (13). And now, NS1 antigen detection kits are commercially available, although it cannot differentiate DENV serotypes. Some studies suggested that different diagnostic methods should be selected according to patient’s onset time: detections combined DF-RNA with NS1 will be more suitable for acute phase (days 1–4), and detections combined DF-NS1 with IgM could increase the detection rate for early convalescence (days ≥5) (11).
According to Biochemical data, liver injury was a significant risk factor for DENV infections (36). In the DF group, AST, ALT, CK, and CRP increased significantly (P<0.01), and AST levels are higher than ALT levels, compared with the OFI group. During dengue virus infections, the pathogenesis of liver involvement is still poorly understood (37). Several mechanisms may be involved in dengue virus inducing liver cell apoptosis. These include direct cytopathic effects of virus or host immune response on liver cells, circulatory compromise, and/or hypoxia caused by hypotension or localized leakage inside the liver capsule and the influence of cellular and humoral immune factors in the liver (37,38). The prominent manifestations were thrombocytopenia (occurring in 28.07% of DF group, compared to 5.18% of OFI group) and leucopenia (occurring in 43.27% of DF group and 3.63% of OFI group). Dengue virus caused DF and DHF, and the major pathophysiological hallmark was increased vascular permeability. In DHF, following plasma leakage caused by increased vascular permeability, hypovolemic shock occurred. Constant hematological abnormalities occurring in DHF and often include bone marrow suppression, leucopenia, and thrombocytopenia (39).
After the severe outbreak in 2014, the government paid more attention to the early detection of imported cases, early mosquito control, and the quarantine of every suspicious case (23). Our data showed that the morbidity of the DF from 2015 to 2018 significantly decreased compared to 2014. Up to date, dengue infection occurred in 2016 and 2017 were less, but an uptrend appeared in 2018 (40). However, because of the subtropical zone, substantial floating population, terrible community environmental conditions, we need to pay more attention to the preventive measures of the DF. On the one hand, there is not an effective anti-DENV vaccine formulation approved for use in humans due to the variety of serotypes and complicated pathogenesis (41). Relevantly, Sanofi Pasteur dengue vaccine Dengvaxia has now been licensed in a few countries, but it recorded poor efficacy in dengue naïve individuals during phase III evaluation (1), which lacks the critical dengue T cell epitopes of the nonstructural region and dengue NS1 that both play vital roles in providing protection against dengue (42-44).
On the other hand, treatment is usually based on symptoms and is performed through medical support since there is no antiviral drug available for dengue. Therefore, the primary way to avoid DF is that community residents, including the individual, need to take protective measures, including clothing, repellents, insecticide-treated mosquito nets, and household fixtures, to avoid biting by mosquitoes. Simultaneously, maintenance of environmental hygiene is considered to be an effective way of dengue prevention and control, including “cleanup” campaigns, regular container emptying and cleaning, installation of water supply systems, solid waste management, and so on (12). Elimination of the breeding places of the mosquitoes, the use of larvicides, and the use of ultralow-volume aerosolized adulticides are the critical point for preventing and controlling DFs (45). The government needs to publicize the knowledge of DF in the community. The participation by the community can be of immense importance in winning the battle against vector mosquitoes (2,3).
Meanwhile, the detection and diagnosis of dengue infection should be strengthened with the identification of viral genomic RNA, antigens, or antibodies. Additionally, the communication and cooperation between the quarantine authorities and the tourism authorities should be enhanced to guarantee no more imported cases. Last but not least, it is significant to develop an alternative dengue vaccine candidate who would enable higher efficacy and applicability to a broader group of subjects, including infants and naïve populations.
Acknowledgments
The authors acknowledge support from the Department of Laboratory Medicine, Nanfang Hospital, Southern Medical University, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Medical Ethics Committee of Nanfang Hospital, Southern Medical University.
References
- Khetarpal N, Khanna I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J Immunol Res 2016;2016:6803098. [Crossref] [PubMed]
- Wu JY, Lun ZR, James AA, et al. Review: Dengue Fever in mainland China. Am J Trop Med Hyg 2010;83:664-71. [Crossref] [PubMed]
- WHO. Available online: (accessed 21.01.13).http://www.who.int/denguecontrol/en/index.html
- Luz PM, Vanni T, Medlock J, et al. Dengue vector control strategies in an urban setting:an economic modelling assessment. Lancet 2011;377:1673-80. [Crossref] [PubMed]
- Luo L, Liang H, Jing Q, et al. Molecular characterization of the envelope gene of dengue virus type 3 newly isolated in Guangzhou, China, during 2009-2010. Int J Infect Dis 2013;17:e498-504. [Crossref] [PubMed]
- Jing QL, Yang ZC, Luo L, et al. Emergence of dengue virus 4 genotype II in Guangzhou, China, 2010: Survey and molecular epidemiology of one community outbreak. BMC Infect Dis 2012;12:87. [Crossref] [PubMed]
- Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol 2006;80:11418-31. [Crossref] [PubMed]
- Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annual Review of Immunology 2011;29:587-619. [Crossref] [PubMed]
- Saxena P, Dash P.K, Santhosh S.R, et al. Development and evaluation of one step single tube multiplex RT-PCR for rapid detection and typing of dengue viruses. Virol J 2008;5:20. [Crossref] [PubMed]
- Guzmán MG, Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis 2004;8:69-80. [Crossref] [PubMed]
- Chen X, Chen R, Gu WS, et al. Clinical Evaluation of Dengue RNA, NS1, and IgM for Diagnosis of Dengue in Southern China. J Med Virol 2016;88:28-34. [Crossref] [PubMed]
- Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010;8:S7-16. [Crossref] [PubMed]
- Neeti M, Shailendra MT, Vineeta K, et al. Comparison of NS1 Antigen and Antibody Detection Method in Early Diagnosis of Dengue Infection. International Journal of Science and Research 2319-7064.
- Nawa M, Yamada KI, Takasaki T, et al. Serotype-cross-reactive immunoglobulin M responses in dengue virus infections determined by enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2000;7:774-7. [PubMed]
- Hunsperger EA, Yoksan S, Buchy P, et al. Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 Antigen and Anti-dengue virus IgM antibody. PLOS Negl Trop Dis 2014;8:e3171. [Crossref] [PubMed]
- Zhang FC, Zhao H, Li LH, et al. Severe dengue outbreak in Yunnan, China, 2013. Int J Infect Dis 2014;27:4-6. [Crossref] [PubMed]
- Yang L, Chen Y, Yan HC, et al. A survey of the 2014 dengue fever epidemic in Guangzhou, China. Emerging Microbes and Infections 2015;4:e57. [Crossref] [PubMed]
- Xiao JP, He JF, Deng AP, et al. Characterizing a large outbreak of dengue fever in Guangdong Province, China. Infect Dis Poverty 2016;5:44. [Crossref] [PubMed]
- Murray NE, Quam MB, Wilder Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013;5:299-309. [PubMed]
- Lu L, Lin H, Tian L, et al. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009;9:395. [Crossref] [PubMed]
- Li TG, Yang ZC, Luo L, et al. Dengue Fever Epidemiological Status and Relationship with Meteorological Variables in Guangzhou, Southern China, 2007-2012. Biomed Environ Sci 2013;26:994-7. [PubMed]
- Li Z, Yin W, Archie C, et al. Spatiotemporal analysis of indigenous and imported dengue fever cases in Guangdong province, China. BMC Infect Dis 2012;12:132-9. [Crossref] [PubMed]
- Cheng Q, Jing QL, Robert C.S, et al. Climate and the Timing of Imported Cases as Determinants of the Dengue Outbreak in Guangzhou, 2014: Evidence from a Mathematical Model. PLoS Negl Trop Dis 2016;10:e0004417. [Crossref] [PubMed]
- Wang L, Wang Y, Jin S, et al. Emergence and control of infectious diseases in China. Lancet 2008;372:1598-605. [Crossref] [PubMed]
- Hadinegoro SR, Arredondo-García JL, Capedingetal MR. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015;373:1195-206. [Crossref] [PubMed]
- Smith W, Gubler DJ. Dengue vaccines at a crossroad: despite modest efficacy, a newly developed vaccine may be key for controlling dengue. Science 2015;350:626-7. [Crossref]
- Witayathawornwong P. DHF in infants, late infants and older children: a comparative study. Southeast Asian J Trop Med Public Health 2005;36:896-900. [PubMed]
- Guo RN, Lin JY, Li LH, et al. The Prevalence and Endemic Nature of Dengue Infections in Guangdong, South China: An Epidemiological, Serological, and Etiological Study from 2005-2011. PLoS One 2014;9:e85596. [Crossref] [PubMed]
- Mallhi TH, Khan AH, Adnan AS, et al. Clinical-laboratory spectrum of dengue viral infection and risk factors associated with dengue hemorrhagic fever: a retrospective study. BMC Infect Dis 2015;15:399. [Crossref] [PubMed]
- García-Rivera EJ, Rigau Perez JG. Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Publica 2003;13:362-8. [Crossref] [PubMed]
- Vazquez S, Hafner G, Ruiz D, et al. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J Clin Virol 2007;39:194-8. [Crossref] [PubMed]
- Bessoff K, Phoutrides E, Delory M, et al. Utility of a commercial nonstructural protein 1 antigen capture kit as a dengue virus diagnostic tool. Clin Vaccine Immunol 2010;17:949-53. [Crossref] [PubMed]
- Huhtamo E, Hasu E, Uzcategui NY, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol 2010;47:49-53. [Crossref] [PubMed]
- Hu D, Di B, Ding X, et al. Kinetics of non-structural protein 1, IgM and IgG antibodies in dengue type 1 primary infection. Virol J 2011;8:47. [Crossref] [PubMed]
- Hu D, Scott RF, Johnny XH, et al. Comparison of Surface Plasmon Resonance, Resonant Waveguide Grating Biosensing and Enzyme Linked Immunosorbent Assay (ELISA) in the Evaluation of a Dengue Virus Immunoassay. Biosensors 2013;3:297-311. [Crossref] [PubMed]
- Wahid SF, Sanusi S, Zawawi MM, et al. A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J Trop Med Public Health 2000;31:259-63. [PubMed]
- Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg 2006;100:608-14. [Crossref] [PubMed]
- Trung DT. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg 2010;83:774-80. [Crossref] [PubMed]
- Srichaikul T, Nimmannitya S. Hematology in dengue and dengue hemorrhagic fever. Baillieres Best Pract Res Clin Haematol 2000;13:261-76. [Crossref] [PubMed]
- Liu ZQ. Information report on dengue fever in Guangzhou. Guangzhou Health and Family Planning Commission 2018, 17.
- Amorim JH, dos Santos Alves RP, Bizerra R, et al. Antibodies are not required to a protective immune response against dengue virus elicited in a mouse encephalitis model. Virology 2016;487:41-9. [Crossref] [PubMed]
- Weiskopf D, Sette A. T-cell immunity to infection with dengue virus in humans. Front Immunol 2014;5:93. [Crossref] [PubMed]
- Modhiran N, Watterson D, Mulleretal DA. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015;7:304ra142. [Crossref] [PubMed]
- Beatty PR, Puerta-Guardo H, Killingbeck SS, et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015;7:304ra141. [Crossref] [PubMed]
- Massad E, Coutinho FA. The cost of dengue control. Lancet 2011;377:1630-1. [Crossref] [PubMed]