
A novel timesaving and semiquantitative method for radionuclide hepatobiliary scintigraphy for suspected biliary atresia
Introduction
Although biliary atresia (BA) and infant hepatitis syndrome (IHS) are the two major causes of infant persistent jaundice, the treatment and prognosis of the two conditions are quite different. Approximately 30% of IHS cases progress to cirrhosis if the patients do not receive proper treatment. Meanwhile, BA requires immediate surgery or else patients experience liver failure and death (1). Therefore, identifying BA as soon as possible is particularly important. Given this need, finding a simple, fast, and practical non-invasive method would have substantial clinical value in the differential diagnosis of BA. A commonly used non-invasive method for BA diagnosis is 99mTc-EHIDA HS (2), although the routine use of 24-h delay imaging is tedious and time-consuming, and several studies have reported its negative affect on diagnostic accuracy (3). We therefore performed a retrospective analysis to investigate the diagnostic efficiency of a novel timesaving and semiquantitative hepatobiliary scintigraphy (HS) method on BA diagnosis with the aim to potentially reduce the imaging time needed to reach a correct diagnosis.
Methods
Clinical data
The data of 185 children with persistent jaundice who had undergone radionuclide 99mTc-EHIDA HS at the First Affiliated Hospital of Guangxi Medical University in China from June 2014 to April 2018, were collected and used in this study. The patient group comprised 113 males and 72 females with an age ranging from 24 to 196 days and an average age of 69.5 days. The mean age of the BA group was 72.49 days, while it was 66 days in the IHS group, with no statistical difference between the age of the two groups. The inclusion criteria were the following: (I) history of jaundice for 2 weeks or more; (II) light yellow or white clay stool color; (III) abnormal liver function, with or without liver enlargement, spleen size and texture changes. The exclusion criteria were the following: (I) incomplete clinical data or incomplete imaging data; (II) breast milk jaundice, jaundice caused by congenital choledochal cyst, toxic liver disease caused by liver tumors, and toxic drugs. According to surgical evaluation and pathological diagnosis, the patients were divided into two groups: the BA group (n=99) and the IHS group (n=86). Both groups underwent 99mTc-EHIDA HS before surgery.
HS method
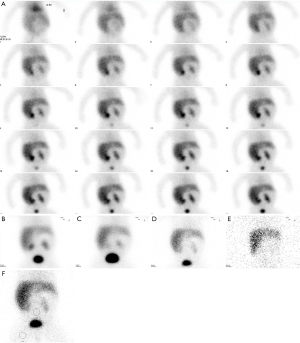
99mTcO4 solution for injection was eluted by Mo technetium generator (Beijing Atomic High Technology Co. Ltd., Beijing, China). An EHIDA kit was provided by the Jiangyuan Pharmaceutical Factory of Jiangsu Institute of Atomic Medicine with a labeling rate ≥95%. The instrument used to perform HS was a GE Infinia dual probe SPECT instrument with a low-energy, high-resolution parallel-hole collimator, an energy peak of 140 Kelvin, and a window width of 20%. The infants were fasted for 2–6 h before HS, and the intravenous infusion channel was prepared in the morning of the day in which HS was performed. A 10% chloral hydrate solution (5 mg/kg) was orally administered 30 min before the injection of the imaging agent. The infants were placed in a supine position, and the imaging field included the liver, heart, intestine, and bladder. Planar radionuclide HS was performed immediately after an intravenous injection of 99mTc-labeled diethylacetanilide-iminodiacetic acid (99mTc-EHIDA) (11.1 MBq/kg). The matrix used was 128×128 pixels, the magnification was 1.0, and a continuous dynamic acquisition was performed for 20 min with a velocity of 1 frame/min. A subsequent acquisition was performed using a matrix of 256×256 pixels under a magnification of 1.0 at 1, 2, 6, and 24 h after injection. Images were obtained in other positions of the instrument to determine the radioactive image area, if necessary. Data were collected using the counting method, and a decision was taken separately by two skilled nuclear medicine physicians. The first frame of dynamic acquisition represented the starting point (500–1,000 k), and those without functional imaging of the gall bladder and/or bowels at 6 or 24 h were considered as BA (recorded as positive). In contrast, functional imaging of the gall bladder and/or bowels indicated the presence of IHS (recorded as negative).
Calculation of 6-h I-B by semi-quantitative HS method
The circular region of interest of the same size as the upper part of the right thigh was delineated in the abdomen around the umbilical cord (avoiding both the kidneys and bladder) on the static images of the hepatobiliary scan in each child at 6 h. The difference in radioactivity uptake was calculated as follows: 6-h I-B = intestinal average radiation uptake value (abdominal periumbilical radiation uptake value) − background average radiation uptake value (right thigh upper part average radiation uptake value).
Statistical analysis
Statistical analysis was performed using SPSS22.0 statistical software. The measurement data in accordance with the normal distribution were expressed as
Results
Comparison of 6-h I-B between the two groups and determination of the diagnostic BA boundary point
The numerical value of 6-h I-B in the BA and IHS group was 2.99±2.04 (counts/pixel) and 6.52±5.14 (counts/pixel), respectively, with the difference being significant (P<0.01). The AUC of BA diagnosed by 6-h I-B was 0.768, the 95% confidence interval was 0.701–0.834, with a P<0.01, demonstrating a good diagnostic value for BA. The results of the receiver operating characteristic (ROC) curve analysis showed that the maximum approximate (Youden) index of 6-h I-B was 5.5 (counts/pixel). Therefore, the threshold for BA diagnosis was set at 6-h I-B <5.5 (counts/pixel).
Comparison between the diagnostic efficacy of 6-h hepatobiliary static imaging semi-quantitative method and conventional 24-h HS visual method
No significant difference was observed in the sensitivity, specificity, accuracy, PPV, and NPV between 6-h hepatobiliary static imaging and conventional 24-h HS (χ2=0.479, 2.389, 0.602, 0.442, and 0.00026, respectively). The 6-h hepatobiliary static imaging resulted in the same diagnostic efficacy as the conventional 24-h HS (Figure 1). The results are shown in Table 1.
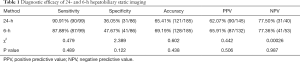
Full table
Discussion
BA is one of the most common congenital malformations of the digestive tract in the neonatal period. Kasai portoenterostomy surgical treatment performed within 60 days in children with BA allow the possibility of normal bile drainage. If the surgery is not performed within 90 days, it generally progresses into liver cirrhosis and liver failure in which case it is difficult to recover a normal condition. However, it is also difficult to distinguish BA from jaundice caused by other diseases such as IHS which does not need operation. Several studies using a large sample size reported the sensitivity (84.6–100%) and specificity (61.1–88.6%) for HS in diagnosing BA (4-6). A recent meta-analysis reported a pooled sensitivity of 98.7% and a pooled specificity of 70.4% (7). The Danish Health Authority states that HS should be performed within three days after conjugated hyperbilirubinemia has been detected (8). Since this non-invasive imaging modality functions as a gate keeper at a very early diagnostic stage of conjugated hyperbilirubinemia in Denmark, our aim was to estimate the high sensitivity and NPV of HS in excluding BA, to avoid more invasive first-line diagnostic procedures, such as liver biopsy in patients with obstructed bile ducts. Routine 24-h radionuclide HS takes time and requires repeated sedation, and, when the condition is particularly serious, many hidden dangers may be present. Moreover, due to decay of the 99mTc radiation, image information decreases after 24 h, thus, it is difficult to discern if the excretion has remained low or increased only slightly (3). Therefore, the quality of 24-hour-delayed imaging is not good, and the misjudgment rate is high. Despite this flaw, it is still crucial to diagnose BA as early and as accurately as possible.
One study reported that (9) the diagnostic efficacy for BA was compared among plane imaging 4–6 h after imaging agent injection, planar imaging 4–6 h after imaging agent injection plus tomography, and tomography 4–6 h after phenobarbital injection, with the sensitivity of the three techniques being found to be identical. Moreover, the need and significance of 6-h HS with 99mTc-EHIDA was emphasized when the specificity of plane imaging 4–6 h after imaging agent injection plus tomography was equivalent to, or even better than, the specificity reported in the literature for phenobarbital intervention. In this case, delayed imaging at 24 h is usually not necessary, indicating the importance of radionuclide 99Tcm-EHIDA 6-h HS.
Since the initial and final dynamic images of scanning provide some information on whether the gallbladder or intestinal tract should be imaged, we hypothesized that eliminating the starting dynamic acquisition and the cumbersome follow-up static acquisition process could reduce the acquisition time and simplify the examination process without affecting the quality of interpretations. Accordingly, our pilot study aimed to retrospectively assess the feasibility of a timesaving and semiquantitative technique of HS for suspected BA without a loss of diagnostic accuracy. Our retrospective study of an HS method for suspected BA relying only on 6-h static plane imaging resulted in a near perfect agreement with the conventional 24-h HS diagnostic accuracy, which is consistent with relevant reports (3). Radionuclide hepatobiliary static imaging at 6-h after injection of 99mTC-EHIDA should replace the conventional 24-h radionuclide HS; this would not only shorten the examination time to 1/4 of the original imaging time but it also simplify the examination procedure, saving dozens of minutes of dynamic imaging process and subsequent multiple static imaging. The problem that children are prone to postural displacement due to the lack of cooperation in the process of multiple examinations can be solved to a certain extent by this method, and more importantly, more time would be gained for the early diagnosis and treatment of BA.
The concept of reducing the imaging time for nuclear studies is not new, and has previously been largely emphasized in nuclear cardiology. However, using this 6-h I-B parameter to evaluate the results of HS has been discussed here for the first time. Moreover, the biggest difference between our 6-h HS and that of other 6-h HS studies is that other’s 6-h HS only removes the delayed 24-h static imaging, while the rest of the traditional imaging process remains the same. In our 6-h HS study, dozens of minutes of dynamic imaging process and subsequent tedious static acquisition process, such as 1-, 2- or 3-h imaging were removed during the traditional start-up. Our method only requires the 6-h static image to calculate the 6-h I-B parameters. It is thus a new, simplified, timesaving, semiquantitative method, which does not need sedation, since only a few minutes are required to collect an image. It can not only maintain good diagnostic efficiency, but it also greatly shortens the examination time and improves the examination efficiency. With the aim of controlling medical expenses, a shift to such technical innovation to reduce the costs and to obtain more patient-centered outcomes appears to be the future of imaging (10). Financial officers at hospitals also seek to maximize technological capital investments by redistributing older equipment to meet the clinical demands imposed by financial and spatial restrictions (11). Thus, in a nuclear medicine department, particularly in smaller facilities that may only have one gamma camera or SPECT, reducing camera time could more easily allow the performance of such an approach, with less effect on the scheduled workflow.
This gained time might be used for a potential ventilation or perfusion scan, renal scan, myocardial perfusion study, or even a bone scan to be performed in an interposed manner as these studies require 6-h of imaging time, resulting in a much more efficient use of technologists and scanner resources. In this way, a cumulative, more cost efficient, increased throughput of studies could be performed; this could be particularly helpful in a small facility with only a single service rate-limiting gamma camera or SPECT. Of course, technologists should demonstrate a logistical skill that allows patients undergoing other examinations to be adequately prepared for hepatobiliary scanning.
Some studies have reported divergent results regarding the age of BA patients compared with the age of patients with intrahepatic cholestasis (12,13). The BA patients in our study were approximately of the same age as the other patients at the time the HS was performed. A large retrospective study carried out by Kvist et al. at the Rigshospitalet found a median age at the time of Kasai operation of 59 days (14), and our study is essentially in accordance with this. Moreover, no significant difference in age between the two groups was found in our study. Age at disease manifestation or gestational age does not seem to be a useful parameter to support the suspected BA in cholestatic infants (15,16).
The phenobarbital test is a classical method in HS for decreasing the false positive rate and increasing the specificity of BA diagnosis. However, we did not perform the phenobarbital test based on several considerations. First, the opportunity for an optimal treatment is more easily lost because the test is longer and more tedious, particularly when regular 24-h imaging does not show the gall bladder and intestine, and thus, a second phenobarbital test is necessary. Second, a known side effect of phenobarbital is liver damage, and its application in liver disease is based on the condition of the patient. In our study, most infants had a high blood bilirubin level and severe liver damage, and clinicians were reluctant to administer phenobarbital in such patients.
Despite the encouraging results obtained, our study possesses some limitations. First, the data were obtained from a single center, and our analysis was performed in a retrospective manner by nuclear physicians from the same group providing initial interpretations. Thus, prospective reproducibility in other, ideally multicenter, environments should be further investigated. We also emphasize that laparotomy with antegrade cholangiography should not be delayed in cases patients around 90 days of age. Interestingly, some studies found that combining HS with SPECT/CT resulted in an increase in the specificity and accuracy of diagnosing BA (9). We believe that adding SPECT/CT in cases of no drainage or suspected atypical drainage could be a useful tool for evaluating the non-BA origin. If the use of additional time does not compromise the precarious health of the patients, other less invasive imaging modalities, such as magnetic resonance cholangiopancreatography, can be considered.
Conclusions
The timesaving and semiquantitative method of 6-h HS evaluated in this study had the same high sensitivity and NPV as the traditional 24-h HS in the diagnosis of persistent jaundice. Although the “gold standard” for the diagnosis of IHS and BA, which are difficult to distinguish clinically, is still laparoscopic bile duct exploration or surgery, our method showed high diagnostic efficacy, good repeatability, non-invasive modality, simplicity, and short time consumption, allowing an early differential diagnosis of persistent jaundice in infants. However, larger multicenter trials are necessary before this clinical approach can be more widely applied.
Acknowledgments
Funding: This research was supported by the Guangxi Medical and Health Appropriate Technology Development and Application Project (S201669).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As we know, a commonly used non-invasive method for BA diagnosis is 99mTc-EHIDA hepatobiliary scintigraphy, which act as a gate keeper at a very early diagnostic stage of conjugated hyperbilirubinemia. Besides, this is a retrospective study, all the data is obtained in the routine examination, no additional examination is required for the child. So this research article was not required the statement of ethics approval.
References
- Superina R, Magee JC, Brandt ML, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg 2011;254:577-85. [Crossref] [PubMed]
- Hopfer K. Nuclear medicine hepatobiliary imaging (cholescintigraphy). Gastrointest Endosc 2011;74:375-7. [Crossref] [PubMed]
- Guan YX, Chen Q, Wan SH, et al. Effect of different time phases of radionuclide hepatobiliary scintigraphy on the differential diagnosis of congenital biliary atresia. Genet Mol Res 2015;14:3862-8. [Crossref] [PubMed]
- Donia AE, Ibrahim SM, Kader M. Predictive value of assessment of different modalities in the diagnosis of infantile cholestasis. J Int Med Res 2010;38:2100-16. [Crossref] [PubMed]
- Esmaili J, Izadyar S, Karegar I, et al. Biliary atresia in infants with prolonged cholestatic jaundice: diagnostic accuracy of hepatobiliary scintigraphy. Abdom Imaging 2007;32:243-7. [Crossref] [PubMed]
- Shah I, Bhatnagar S, Rangarajan V, et al. Utility of Tc99m-mebrofenin hepato-biliary scintigraphy (HIDA scan) for the diagnosis of biliary atresia. Trop Gastroenterol 2012;33:62-4. [Crossref] [PubMed]
- Kianifar HR, Tehranian S, Shojaei P, et al. Accuracy of hepatobiliary scintigraphy for differentiation of neonatal hepatitis from biliary atresia: systemic review and meta-analysis of the literature. Pediatr Radiol 2013;43:905-19. [Crossref] [PubMed]
- Available online: (13 Feb 2013).www.sst.dk/publ/Publ2004/galdevejsatresi.pdf
- Sevilla A, Howman-Giles R, Saleh H, et al. Hepatobiliary scintigraphy with SPECT in infancy. Clin Nucl Med 2007;32:16-23. [Crossref] [PubMed]
- Shaw LJ. Cost-effectiveness and future implications for cardiovascular imaging. Can J Cardiol 2013;29:350-7. [Crossref] [PubMed]
- Poplin B. Making informed capital investment decisions for clinical technology. Healthc Financ Manage 2011;65:64-8. [PubMed]
- Donia AE, Ibrahim SM, Kader M. Predictive value of assessment of different modalities in the diagnosis of infantile cholestasis. J Int Med Res 2010;38:2100-16. [Crossref] [PubMed]
- Poddar U, Bhattacharya A, Thepa BR, et al. Ursodeoxycholic acid-augmented hepatobiliary scintigraphy in the evaluation of neonatal jaundice. J Nucl Med 2004;45:1488-92. [PubMed]
- Kvist N, Davenport M. Thirty-four years’ experience with biliary atresia in Denmark: a single center study. Eur J Pediatr Surg 2011;21:224-8. [Crossref] [PubMed]
- Joseph U A. Nuclear Hepatology: A Textbook of Hepatobiliary Diseases. J Nucl Med 2010;51:1655-6. [Crossref]
- Mansi L, Landolfi C. G. T. Krishnamurthy and S. Krishnamurthy: Nuclear hepatology: a textbook of hepatobiliary diseases (2nd edition). Eur J Nucl Med Mol Imaging 2011;38:1386-7.


