
Patterns and infection outcomes of bacterial colonization in patients with indwelling abdominal drains for malignant ascites
Introduction
Background
Ascites is a common cancer-related complication in patients with advanced malignancy. Clinically significant amount of ascites causes considerable symptom burden including abdominal distention, pain, dyspnea, nausea, vomiting, early satiety, constipation, reduced mobility and problems with the body image (1-4). Diuretic therapy is one of the management options of ascites in cancer patients however evidence regarding the use of diuretics in the context of malignant ascites is weak and controversial (1). The clinical benefit appears to be less in patients with malignant ascites caused by peritoneal carcinomatosis. Many of the advanced cancer patients have ascites that is refractory to medical management. Abdominal paracentesis provides good relief of ascites-related symptoms in 78–90% of patients yet the effects are usually temporary (1,2). Repeated paracenteses many a time are necessitated due to the inevitably re-accumulating ascites and as a result exposing patients to repeated invasive procedures and risks of related complications (5-7). Patients may also wait until substantial re-accumulation of ascites before having another paracentesis to avoid frequent procedures and hospital stays, which could result in detrimental effects on their quality of life (8). Extra burden may also be imposed on their families due to the related frequent hospital admissions (5).
Indwelling abdominal drain for intermittent drainage
For advanced cancer patients with refractory ascites, insertion of indwelling catheters into the abdominal cavity for intermittent drainage can be considered in order to prevent repeated invasive procedures and multiple hospitalizations, thus improving quality of life (3,5-7). These catheters can either be percutaneous catheters inserted directly through the abdominal wall into the peritoneal cavity, for example, pigtail catheters and central venous catheters (CVCs), or catheters with subcutaneous tunnels, for example, PleurX catheters and Tenckhoff catheters (3). The rate of technical success of indwelling catheters insertion was reported to be 100% and the rate of symptom control was 75% to 100% (3,4). A significant improvement in symptom control and quality of life has also been reported in almost all patients (3-7,9-14). In light of these favorable clinical outcomes, insertion of indwelling abdominal drains appears to be a promising option for relieving refractory ascites in patients with advanced malignancy.
Bacterial colonization and drain-related infection
Although the technical safety and clinical efficacy of indwelling abdominal drains for advanced cancer patients with refractory ascites have been demonstrated (3-7,9-25), bacterial colonization and subsequent drain-related infection remain to be common concerns for these types of long-term drains inserted into the peritoneal cavity as in patients with other types of long-term indwelling catheters, for example, Tenckhoff catheters (26), central venous access (27-29) and urinary catheters (30). Colonization of these catheters can lead to resistance to antibiotic treatment because of the formation of bacterial biofilm (31-33). Majority of the previous studies regarding indwelling abdominal drains for ascites focused on technical safety, procedures and feasibility, while some focused on the efficacy of ascites drainage, patient-reported outcomes and quality of life (3). Data concerning bacterial colonization and the subsequent infection outcomes have been very limited. Majority of the previous studies did not mention about performing surveillance ascitic fluid culture (5-7,9,11-15,17-25), therefore the patterns of bacterial colonization in this particular group of patients is still largely unknown. The significance of bacterial colonization in terms of subsequent progression into overt drain-related infection is also yet to be addressed by the currently available literatures.
In this study, the patterns of bacterial colonization and the subsequent infection outcomes of advanced cancer patients on indwelling abdominal drains for intermittent drainage of ascites were investigated. Factors that would be potentially correlating with the development of drain-related infection from bacterial colonization were also analyzed.
Methods
The ascites clinic
The ascites clinic of the Department of Clinical Oncology, Tuen Mun Hospital (“ascites clinic”) has been established to provide out-patient intermittent drainage for advanced cancer patients who have refractory ascites. These patients are either having CVCs inserted by the Clinical Oncologists or pigtail drains inserted by the Interventional Radiologists or Surgeons during prior admissions for symptomatic ascites. The patients are subsequently referred to the ascites clinic with the abdominal drains in-situ to continue intermittent drainage as out-patients. The ascites clinic is a nurse-led clinic in which clinical assessment and drainage are performed by palliative care nurses according to departmental guidelines. Prior to each episode of drainage, physical parameters including body weight and vital signs will be assessed. Performance status will be evaluated using the Palliative Performance Scale (PPS) (34). Physical symptoms will be assessed in each session using the Edmonton Symptom Assessment System (ESAS) (35,36), with an 11-point Likert scale for abdominal distention fitting into the last assessment item–“other problems” of the ESAS. Inspection of the drain insertion site for signs of infection will be performed prior to each episode of drainage. All drainage procedures are done under aseptic technique with the abdominal drain connected to a bedside bag for free drainage by gravity, while the patient is lying in a supine position. After each drainage, a new sterile spigot will be applied to the abdominal drain and dressing of the drain site will be performed. Education will be provided by nurses to patients and carers on proper care of the drain and monitoring of signs of infection. Home-care nursing support is available for patients with difficulties in self-care of the drains. Patients and relatives will also be given instructions for not having self-drainage at home to avoid inappropriate drainage procedures causing undesirable complications. Ascitic fluid specimens for cell count, Gram stain and bacterial culture will be collected routinely every week whenever possible during ascites clinic follow-up as surveillance. Patients with positive ascitic fluid culture results will be assessed by the duty Clinical Oncologist in the ascites clinic and conservative approach will be adopted if there are no clinical features of infection. All the clinical information obtained during each ascites clinic visit will be documented in a standardized clinical assessment form as a routine clinical practice. Majority of the patients under the service of the ascites clinic will have assessment and drainage performed by nurses once to twice every week depending on the clinical need of ascites drainage and at the same time having routine clinic assessment by the duty Clinical Oncologist at least once every 4 weeks.
Study design and patients
This study was a retrospective cohort study. All consecutive advanced cancer patients with newly inserted indwelling abdominal drains and who were under the service of the ascites clinic of our institution for intermittent drainage between January 2011 and March 2018 were screened for study eligibility. Patients with positive surveillance ascitic fluid culture without immediate drain-related infection were included in the final analysis. Patients who had temporary insertion of abdominal drain for short-term drainage during a single admission episode were not included. Those patients who did not have any proper clinical assessment completed and those without any attempts of drainage in the ascites clinic were also excluded.
Data collection
All data were collected from the date of indwelling abdominal drain insertion till the date of end-point or censoring. Clinical information was prospectively collected using the standardized clinical assessment forms as part of the routine clinical practice of the ascites clinic. These clinical assessment forms and other medical records of the patients were retrospectively reviewed. Patients’ demographic data and information regarding the cancer diagnosis and co-morbidities were collected. Clinical information including performance status, physical parameters and symptoms, drain condition and details of each drainage episode were also retrieved. All ascitic fluid cell count, Gram stain and culture, drain-site wound swab culture and blood culture results that were available during the study period were extracted from the electronic medical records. Infection outcomes and survival outcomes were also obtained.
Bacterial colonization, infection outcomes and end-points
Bacterial colonization of the abdominal drain was defined as having positive surveillance ascitic fluid bacterial culture in the absence of any clinical features of infection. Drain-site cellulitis and drain-related peritonitis were both included as infection outcomes. Drain-site cellulitis was defined as the presence of drain-site inflammation, pain and discharge. Drain-related peritonitis was defined as the condition presenting with clinical features of sepsis together with abdominal symptoms and signs or positive ascitic fluid culture that could be accountable for the sepsis. Fever or sepsis without other demonstrable foci and infected ascitic fluid on inspection, which was defined as turbid or malodorous fluid, were also considered as infection outcomes. Patients without any of these clinical features but were initiated on antibiotics, with or without drain removal, based on positive ascitic fluid culture results were also included as having drain-related infection and were categorized as physician-diagnosed drain-related infection. The infection end-point was defined as the date of diagnosis of drain-related infection while patients without infection outcomes were censored at the date of death or last follow-up or the date of premature removal of indwelling abdominal drain. Premature removal of indwelling abdominal drain was defined as removal of drain due to conditions other than infection and without re-insertion within 2 weeks. Infection-free survival was calculated from the date of first documented positive ascitic fluid culture till the date of infection outcomes.
Statistical analysis
Descriptive analysis was performed with categorical data presented as counts and percentages. Continuous data were described using median, minimum and maximum values. Time-to-event data were described using the Kaplan-Meier method. Categorical variables were compared by the Pearson chi-square test, or the Fisher’s exact test when the expected value in any of the cells in the contingency table was below 5, while continuous variables were compared by the independent samples T test and Mann Whitney U test. Two-sided P values of less than 0.05 were considered as statistically significant. Multivariate analysis using binary logistic regression was applied to test the independent significance of different covariates. All variables trending towards significance in univariate analyses (P<0.10) were included in the multivariate analysis and were entered in a forward stepwise approach. Statistical analysis was performed using the International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS®) Software version 22 (IBM Corporation, Armonk, NY, USA).
Results
One hundred and forty three advanced cancer patients with indwelling abdominal drains were under the service of the ascites clinic between January 2011 and March 2018. A total of 69 patients (48.3%) developed bacterial colonization without immediate infection. All of them were eligible for the study and included in the final analysis.
Demographic characteristics
The median age at indwelling abdominal drain insertion was 63.3 years (range, 35.5–93.2 years). The male to female ratio was 1.16:1. The most common cancer diagnosis was hepatocellular carcinoma (HCC), comprising 30.4% of the population. Majority of patients (76.8%) had metastatic disease and 68.1% had documented peritoneal metastases. Cirrhosis was a common co-morbid condition and was present in 27.5% of the study population (Table 1).
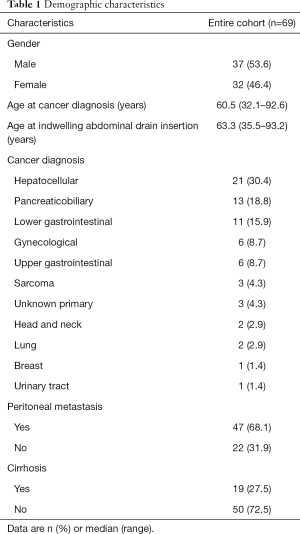
Full table
Ascites drainage
CVC was the most common type of indwelling abdominal drain (75.4%) inserted initially, while 17 patients (24.6%) had pigtail drains. Seven patients (10.1%) had their drains changed prior to development of bacterial colonization, with 76.8% having CVCs and 23.2% having pigtail drains as the last type of indwelling abdominal drain in-situ before first documentation of positive ascitic fluid culture. The details of intermittent drainage performed in the ascites clinic prior to bacterial colonization were outlined in Table 2.
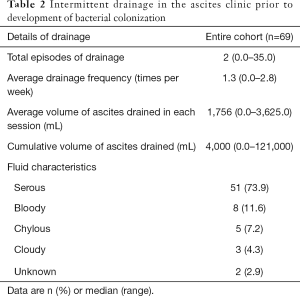
Full table
Baseline physical condition
Upon the first attendance in the ascites clinic, the cohort of patients were having a median PPS level of 60% (range, 40–80%). The median Charlson Comorbidity Index (CCI) (37) was 6 (range, 2–11) and the median post-drainage ESAS was 14/100 (range, 0–41). None of these patients were having concurrent chemotherapy during the ascites clinic follow-up and the median duration free from chemotherapy was 3.0 months (range, 0.5–58.2 months) at the time of indwelling abdominal drain insertion. One patient with HCC was having Sorafenib during the period when he was under the service of the ascites clinic.
Colonization
The median duration from drain insertion to the development of bacterial colonization was 18.0 days (range, 5–159 days). With regard to the first positive surveillance ascitic fluid culture, 79.7% (55 patients) had one type of bacteria isolated from the ascitic fluid, 8.7% had two and 11.6% had three or more. Thirteen percent (9 patients) had their ascitic fluid specimens being positive for Gram stain. Twenty patients (29.0%) had at least one type of bacteria showing heavy growth. The most predominant type of bacteria isolated was Staphylococci—other than Methicillin-resistant Staphylococcus aureus (MRSA), which was present in 43.5% of the first positive ascitic fluid specimens. The second most common type being Enterococci—other than Vancomycin-resistant Enterococci (VRE), which was present in 17.4% of the specimens and Diphtheroid bacilli ranked third (14.5%) (Table 3).

Full table
Summarizing all the surveillance ascitic fluid culture results, 21 patients (30.4%) had one positive ascitic fluid culture documented since the insertion of indwelling abdominal drain and before the infection end-point, 15 patients (21.7%) had two and 33 (47.8%) had three or more positive ascitic fluid culture prior to the infection end-point. The highest number of types of bacteria that were isolated from a single ascitic fluid specimen was 5 and it occurred in 1 patient (1.4%). Twenty two patients (31.9%) have ever had at least one positive Gram stain result and 34 patients (49.3%) have ever had at least one specimen showing heavy growth of bacteria. Staphylococci–other than MRSA remained the most common type of bacteria and it was present in at least one of the surveillance specimens in 53.6% of patients. Diphtheroid bacilli and Enterococci–other than VRE ranked second and third and were present in at least one ascitic fluid specimen in 36.2% and 31.9% of patients respectively. Table 4 shows the details of the bacterial colonization patterns.

Full table
Infection outcomes
Thirty patients (43.5%) developed drain-related infection subsequently and the median time from first documentation of bacterial colonization to development of infection was 14.5 days (range, 3–117 days). The incidence rate of drain-related infection after development of bacterial colonization was 1.78 per 100-catheter days. The median infection-free survival was 45 days with a 95% confidence interval (95% CI) of 17.3–72.7, while the 1-month infection-free survival was 54.4%.
Among the 30 patients who developed drain-related infection, 5 (16.7%) developed peritonitis, corresponding to 7.2% of the entire study population (Table 5). MRSA was isolated from the ascitic fluid specimens in 3 patients during the peritonitis episode and Staphylococcus aureus was isolated in 2 patients. Six patients (20.0%) developed drain-site cellulitis and 3 of them occurred concurrently with other infection conditions, of which 2 were infected ascitic fluid on inspection and the remaining one was peritonitis (Table 5). Eight patients (26.7%) were noted to have infected ascitic fluid on inspection (Table 5), which were characterized by either turbidity or malodor of the ascitic fluid without clinical features of peritonitis. Fever or sepsis without other demonstrable foci occurred in 3 patients (10.0%) while having the indwelling abdominal drains in-situ (Table 5). Eleven patients (36.7%) were considered as having physician-diagnosed drain-related infection (Table 5). Seven of them were prescribed with intravenous broad-spectrum antibiotics and 4 of them were initiated on oral antibiotics. Four patients (13.3%) died of drain-related infection and all of them were having peritonitis. There was no mortality in other categories of drain-related infection.

Full table
Excluding the 3 patients with isolated drain-site cellulitis, 24 out of the remaining 27 patients had ascitic fluid culture results available during the infection episodes. Enterococci—other than VRE and Staphylococci—other than MRSA were the two predominant types of bacteria isolated from the ascitic fluid, both of which were present in 45.8% of patients. Staphylococcus aureus was more common than Coagulase negative staphylococcus within the Staphylococci group. Majority (54.2%) of these ascitic fluid specimens had overlapping types of bacteria with those isolated in the first positive surveillance ascitic fluid culture. Almost all (95.8%) of these ascitic fluid specimens obtained during the infection episodes yielded types of bacteria that were ever present in at least one of the previous surveillance ascitic fluid culture. Table 6 shows the details of the ascitic fluid culture results during the infection episodes.
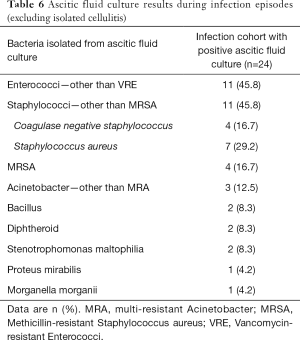
Full table
Factors correlating with drain-related infection
In univariate analyses, a decrease in body mass index (BMI) (P=0.03) and having 3 or more episodes of drainage in the ascites clinic before documentation of bacterial colonization (P=0.03) were statistically significant factors correlating with development of drain-related infection. While weight loss (P=0.08), HCC as the cancer diagnosis (P=0.06) and duration on abdominal drain more than 20 days before first positive surveillance ascitic fluid culture (P=0.1) were factors showing trends towards statistical significance. With regard to the demographic characteristics and baseline physical conditions, gender (P=0.47), age (P=0.62), baseline CCI (P=0.49), baseline PPS (P=0.84), baseline ESAS and cirrhosis as co-morbidity (P=0.42) were not found to be correlating with infection outcomes. The type of indwelling abdominal drain inserted was not correlating with drain-related infection neither (P=1.00). Changing to a new indwelling abdominal drain after documentation of bacterial colonization did not appear to alter the infection outcomes [odds ratio (OR) 0.97, P=0.65]. However only 7 patients (10.1%) had their drain changed after documentation of bacterial colonization.
Regarding the surveillance ascitic fluid culture results, presence of Escherichia coli (P=0.04) and Bacillus species (P=0.04) in any of the surveillance ascitic fluid culture were significantly correlating with infection outcomes. The presence of Staphylococci—other than MRSA in any of the surveillance ascitic fluid culture has also shown a trend towards statistical significance (P=0.09). However, Gram stain positive results (P=0.12), heavy growth of bacteria (P=0.34), having 3 or more types of bacteria in a single ascitic fluid culture specimen (P=0.12) and the ascitic fluid white blood cell count (P=0.35) were not significantly correlating with drain-related infection.
In multivariate analysis, HCC as cancer diagnosis (OR 8.85, 95% CI: 1.86–42.07, P=0.006) and decrease in body weight (OR 1.20, 95% CI: 1.02–1.42, P=0.03) remained significant factors that positively correlating with infection outcomes. Table 7 shows the results of multivariate analysis.

Full table
Discussion
There have been limited data in previous literatures regarding the patterns of bacterial colonization and the subsequent infection outcomes in patients on indwelling abdominal drains for intermittent drainage of malignant ascites. Belfort et al. (10) investigated the use of implantable silastic drain in 17 patients, 8 of them (47.1%) had culture-positive peritoneal fluid but there was only one case of peritonitis reported. Four patients (21.1%) had surveillance ascitic fluid culture performed in the series reported by Fleming et al. (16) and none of these specimens were positive for bacterial growth. In our study, 143 patients were under the service of the ascites clinic during the study period and up to half of the cohort had documented bacterial colonization without immediate infection. Bacterial colonization in patients with indwelling abdominal drains appears to be an alarmingly common condition based on the current data. The median time from indwelling drain insertion to development of bacterial colonization was 18.0 days only, which was much shorter than the reported median survival of patients with malignant ascites (1,38). This implies that bacterial colonization remains an issue that would still affect many of the patients on indwelling abdominal drains for malignant ascites even though they are usually having limited life expectancy.
Similar to the conditions in other types of indwelling catheters (26,28,31), Staphylococci was the most common type of bacteria causing abdominal drain colonization in our cohort. Diphtheroid bacilli and Enterococci were the other two most common types of bacteria isolated in the surveillance ascitic fluid culture. Staphylococci and Diphtheroids are common commensals of the skin and mucosa, while Enterococci is a common pathogen for nosocomial infection. Colonization by these organisms would not be unexpected in patients with percutaneously inserted catheters who have frequent hospital visits for intermittent drainage of ascites. The underlying mechanisms of developing colonization may be similar to the situation in Tenckhoff catheters as proposed by Read et al. (26). Bacterial growth at the skin causes formation of biofilms that adhere to the catheter and tissue surface. Subsequent contiguous spread along the outside of the catheter surface, through the cutaneous exit site into the peritoneal cavity results in contamination of the peritoneal fluid. Bacteria can also spread into the peritoneum transluminally during ascites drainage if the aseptic technique is breached.
The fact that more than 40% of patients with colonization progressed into subsequent drain-related infection in the current study has illustrated this condition to be a clinically significant problem. The median time from colonization to development of infection was only 14.5 days and nearly half of the patients with colonization would progress into drain-related infection within 1 month. Of note, only 5 patients (7.2%) in the entire colonization cohort developed overt peritonitis. However, 4 out of these 5 patients died of the infection episode, which implied an 80.0% case-fatality rate in patients who had drain-related peritonitis. Staphylococci remained the most common type of bacteria isolated during the infection episodes. All except one patient had overlapping types of bacteria isolated from the peritoneal fluid during infection when compared with the surveillance ascitic fluid specimens during colonization.
None of the demographic characteristics nor the baseline physical condition were demonstrated to have correlation with subsequent infection. A decrease in BMI was found to be a significant factor in univariate analysis yet the correlation was not demonstrated in multivariate analysis. Similarly, having 3 or more drainage episodes in the ascites clinic before development of colonization appeared to be a significant factor in univariate analysis but not in subsequent multivariate analysis. Positive Gram stain results, heavy growth of bacteria or high white blood cell count in the surveillance ascitic fluid specimens did not help to predict subsequent infection neither. Presence of Escherichia coli and Bacillus species were significantly correlating with infection outcomes in the univariate analyses. However, none of the bacterial types that were ever present in the surveillance ascitic fluid culture could predict progression from colonization to infection. These negative results could possibly be due to the underpower of the study given the limited population size.
Decrease in body weight and having HCC as cancer diagnosis were the only two factors that significantly correlated with subsequent drain-related infection in multivariate analysis. Decrease in body weight may probably be just a surrogate marker for deterioration in physical condition and nutritional status in patients with advanced malignancy, which as a result could lead to the impairment of the immunity and the susceptibility to infection. HCC seldom progresses with peritoneal carcinomatosis and the ascites in patients with HCC is mainly caused by portal hypertension resulting from extensive liver involvement, porto-venous compression and concomitant cirrhosis (39), while malignant ascites in patients with peritoneal metastasis is largely related to increased vascular permeability and obstruction of lymphatic drainage, resulting in an imbalance between the production and resorption of plasma exudate in the peritoneal cavity (2,39). Ascites in HCC patients appears to resemble the condition in patients with other benign chronic liver diseases. Given the differences in aetiologies of ascites between patients with HCC and peritoneal metastasis, it is plausible that the unique micro-environment inside the peritoneum and ascitic fluid in each condition could result in a different risk of drain-related infection. In one of the largest studies using indwelling tunnelled peritoneal drainage catheter for management of ascites in patients with end-stage liver disease by Solbach et al., bacterial peritonitis was reported to occur in 8.3% of patients (40). The reported risk of peritonitis appears to be slightly higher than that reported in the largest malignancy-associated counter-part by Wong et al. (24), in which the risk of peritonitis was 2.5%. It may be postulated that the risk of drain-related infection is slightly higher in patients with ascites of the chronic liver disease type. However previous literatures did not provide data on direct comparison of the risks regarding drain-related infection between ascites of different disease types.
Studies have been conducted to investigate various methods in reducing catheter colonization. A Cochrane review in 2005 (27) has assessed the effectiveness of antimicrobial impregnation in reducing catheter colonization and catheter-related infection in patients with central venous catheterization. It concluded that catheters with antimicrobial modifications significantly reduced catheter colonization. Antibiotic lock therapy has also been investigated by Zanwar et al. as treatment of colonization to salvage the catheters in patients with CVCs (28). Clearance of the colonization was achieved in 91.0% of patients. Whether these methods are applicable to the situation of indwelling abdominal drains should be further explored by future studies. Yasuda et al. compared the efficacy of different antiseptic solutions in preventing intravascular catheter colonization (29). 0.5% and 1.0% alcohol/chlorhexidine gluconate were found to be superior than 10% povidone iodine. 70% ethyl alcohol and 10% povidone iodine are the two antiseptic solutions that would be used prior to drain insertion in our standard practice. Whether changing to a different antiseptic solution would result in less colonization of the abdominal drains remains a question to be addressed by future studies. Changing to a new abdominal drain after bacterial colonization did not appear to alter the infection outcomes as demonstrated in our study. However, one should be cautious that only a small number of patients (10.1%) had their drain changed after bacterial colonization in the current study, thus limiting the conclusion that can be deduced from the present data.
There are several limitations in our study. Culture of the catheters tips was not performed in the present study. Ascitic fluid culture results may not always be concordant with the colonization inside the catheters. A previous study investigating bacterial colonization of urethral catheters did demonstrate discrepancies between the urine culture and the catheter culture (30). Without removal of drains for culture of the catheters tips, genuine patterns of colonization may not be revealed. Nevertheless, liberal removal of indwelling abdominal drains upon colonization would be undesirable, as this may subject the frail cancer patients to repeated invasive procedures. More equivocal clinical conditions such as infected ascitic fluid on inspection and physician-diagnosed drain-related infection were also included as infection outcomes. The proportion of patients progressing from colonization to drain-related infection may be over-reported. In real-world clinical practice, it may be difficult to differentiate pure colonization from subtle infection. Reporting of these entities may actually represent a more thorough capturing of the spectrum of drain-related infection. Although this is a retrospective study, clinical information was collected prospectively using the standardized clinical assessment forms. Patients were followed up with a relatively regular and frequent schedule in the ascites clinic. Limitations from a retrospective study design should be partly overcome.
Conclusions
In conclusion, bacterial colonization is a significant clinical condition in patients on indwelling abdominal drains for malignant ascites. Subsequent progression into drain-related infection occurs in more than 40.0% of patients. Staphylococci is the most common type of bacteria causing both colonization and subsequent drain-related infection. HCC and decrease in body weight are significant factors that correlate with infection after bacterial colonization.
Acknowledgments
Dr. Chi Sing Frank Wong, the department chief, for his general support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rebecca Yeung and Tai Chung Lam) for the series “Integrating Palliative Medicine in Oncology Care: The Hong Kong Experience”, published in Annals of Palliative Medicine. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2019.09.15). The series “Integrating Palliative Medicine in Oncology Care: The Hong Kong Experience” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: This is a retrospective analysis of charts of patients, informed consent was not considered to be necessary. The patients’ personal data have been secured. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol has been approved by the local institutional review board (New Territories West Cluster Research Ethics Committee, Hospital Authority, Hong Kong). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Becker G, Galandi D, Blum HE. Malignant ascites: Systematic review and guideline for treatment. Eur J Cancer 2006;42:589-97. [Crossref] [PubMed]
- Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res 2009;29:3353-9. [PubMed]
- Stukan M. Drainage of malignant ascites: patient selection and perspectives. Cancer Manag Res 2017;9:115-30. [Crossref] [PubMed]
- Christensen L, Wildgaard L, Wildgaard K. Permanent catheters for recurrent ascites–a critical and systematic review of study methodology. Support Care Cancer 2016;24:2767-79. [Crossref] [PubMed]
- Mercadante S, Intravaia G, Ferrera P, et al. Peritoneal catheter for continuous drainage of ascites in advanced cancer patients. Support Care Cancer 2008;16:975-8. [Crossref] [PubMed]
- Lee A, Lau TN, Yeong KY. Indwelling catheters for the management of malignant ascites. Support Care Cancer 2000;8:493-9. [PubMed]
- Qu C, Xing M, Ghodadra A, et al. The impact of tunneled catheters for ascites and peritoneal carcinomatosis on patient rehospitalizations. Cardiovasc Intervent Radiol 2016;39:711-6. [Crossref] [PubMed]
- Keen A, Fitzgerald D, Bryant A, et al. Management of drainage for malignant ascites in gynaecological cancer. Cochrane Database Syst Rev 2010.CD007794. [Crossref] [PubMed]
- Barnett TD, Rubins J. Placement of a permanent tunneled peritoneal drainage catheter for palliation of malignant ascites: a simplified percutaneous approach. J Vasc Interv Radiol 2002;13:379-83. [Crossref] [PubMed]
- Belfort MA, Stevens PJ, DeHaek K, et al. A new approach to the management of malignant ascites; a permanently implanted abdominal drain. Eur J Surg Oncol 1990;16:47-53. [PubMed]
- Courtney A, Nemcek AA Jr, Rosenberg S, et al. Prospective evaluation of the PleurX catheter when used to treat recurrent ascites associated with malignancy. J Vasc Interv Radiol 2008;19:1723-31. [Crossref] [PubMed]
- Gu X, Zhang Y, Cheng M, et al. Management of non-ovarian cancer malignant ascites through indwelling catheter drainage. BMC Palliat Care 2016;15:44. [Crossref] [PubMed]
- O'Neill MJ, Weissleder R, Gervais DA, et al. Tunneled peritoneal catheter placement under sonographic and fluoroscopic guidance in the palliative treatment of malignant ascites. AJR Am J Roentgenol 2001;177:615-8. [Crossref] [PubMed]
- Stukan M, Leśniewski-Kmak K, Wróblewska M, et al. Management of symptomatic ascites and post-operative lymphocysts with an easy-to-use, patient-controlled, vascular catheter. Gynecol Oncol 2015;136:466-71. [Crossref] [PubMed]
- Akinci D, Erol B, Ciftci TT, et al. Radiologically placed tunneled peritoneal catheter in palliation of malignant ascites. Eur J Radiol 2011;80:265-8. [Crossref] [PubMed]
- Fleming ND, Alvarez-Secord A, Von Gruenigen V, et al. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J Pain Symptom Manage 2009;38:341-9. [Crossref] [PubMed]
- Lungren MP, Kim CY, Stewart JK, et al. Tunneled peritoneal drainage catheter placement for refractory ascites: single-center experience in 188 patients. J Vasc Interv Radiol 2013;24:1303-8. [Crossref] [PubMed]
- Maleux G, Indesteege I, Laenen A, et al. Tenckhoff tunneled peritoneal catheter placement in the palliative treatment of malignant ascites: technical results and overall clinical outcome. Radiol Oncol 2016;50:197-203. [Crossref] [PubMed]
- Narayanan G, Pezeshkmehr A, Venkat S, et al. Safety and efficacy of the PleurX catheter for the treatment of malignant ascites. J Palliat Med 2014;17:906-12. [Crossref] [PubMed]
- Richard HM 3rd, Caldwell DM, Boyd-Kranis RL, et al. Pleurx tunneled catheter in the management of malignant ascites. J Vasc Interv Radiol 2001;12:373-5. [Crossref] [PubMed]
- Rosenberg S, Courtney A, Nemcek AA Jr, et al. Comparison of percutaneous management techniques for recurrent malignant ascites. J Vasc Interv Radiol 2004;15:1129-31. [Crossref] [PubMed]
- Tapping CR, Ling L, Razack A. PleurX drain use in the management of malignant ascites: safety, complications, long-term patency and factors predictive of success. Br J Radiol 2012;85:623-8. [Crossref] [PubMed]
- Knight JA, Thompson SM, Fleming CJ. Safety and effectiveness of palliative tunneled peritoneal drainage catheters in the management of refractory malignant and non-malignant ascites. Cardiovasc Intervent Radiol 2018;41:753-61. [Crossref] [PubMed]
- Wong BC, Cake L, Kachuik L, et al. Indwelling Peritoneal Catheters for Managing Malignancy-Associated Ascites. J Palliat Care 2015;31:243-9. [Crossref] [PubMed]
- Meier M, Mortensen FV, Madsen HH. Malignant ascites in patients with terminal cancer is effectively treated with permanent peritoneal catheter. Acta Radiol Open 2015;4:2058460115579934. [Crossref] [PubMed]
- Read RR, Eberwein P, Dasgupta MK, et al. Peritonitis in peritoneal dialysis: bacterial colonization by biofilm spread along the catheter surface. Kidney Int. 1989;35:614-21. [Crossref] [PubMed]
- Lai NM, Chaiyakunapruk N, Lai NA, et al. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 2016;3:CD007878. [Crossref] [PubMed]
- Zanwar S, Jain P, Gokarn A, et al. Antibiotic lock therapy for salvage of tunneled central venous catheters with catheter colonization and catheter-related bloodstream infection. Transpl Infect Dis 2019;21:e13017. [Crossref] [PubMed]
- Yasuda H, Sanui M, Abe T, et al. Comparison of the efficacy of three topical antiseptic solutions for the prevention of catheter colonization: a multicenter randomized controlled study. Crit Care 2017;21:320. [Crossref] [PubMed]
- Matsukawa M, Kunishima Y, Takahashi S, et al. Bacterial colonization on intraluminal surface of urethral catheter. Urology 2005;65:440-4. [Crossref] [PubMed]
- Raad I, Darouiche R, Hachem R, et al. Antibiotics and prevention of microbial colonization of catheters. Antimicrob Agents Chemother 1995;39:2397-400. [Crossref] [PubMed]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318-22. [Crossref] [PubMed]
- Nickel JC, Ruseska I, Wright JB, et al. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 1985;27:619-24. [Crossref] [PubMed]
- Anderson F, Downing GM, Hill J, et al. Palliative performance scale (PPS): a new tool. J Palliat Care 1996;12:5-11. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [Crossref] [PubMed]
- Watanabe SM, Nekolaichuk C, Beaumont C, et al. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage 2011;41:456-68. [Crossref] [PubMed]
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]
- Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 2007;18:945. [Crossref] [PubMed]
- Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology 1988;8:1104-9. [Crossref] [PubMed]
- Solbach P, Höner Zu Siederdissen C, Taubert R, et al. Home-based drainage of refractory ascites by a permanent-tunneled peritoneal catheter can safely replace large-volume paracentesis. Eur J Gastroenterol Hepatol 2017;29:539-46. [Crossref] [PubMed]


