
Multiple bone metastases: what the palliative care specialist should know about the potential, limitations and practical aspects of radiation therapy
Introduction
Epidemiology
Bone metastases represent a significant health care problem in the cancer population. It is estimated that bone is the third most common site of metastases after lung and liver. Common primary cancers which tend to metastasize to bone include breast cancer, prostate cancer and lung cancer (1). One autopsy series reported that 70% of breast cancer patients had metastases to bone (2).
Diagnosis
Plain X-rays are useful in identifying bone metastases and can help characterize if the metastasis is osteolytic, osteoblastic, or mixed. Plain X-rays can also diagnose pathologic fractures and impending fractures. Plain X-rays are inexpensive and readily accessible. However, plain X-rays may miss the presence of bone metastases.
Bone scans have the highest sensitivity for osteoblastic metastases but may not pick up osteolytic bone lesions (such as seen in multiple myeloma). Bone scans allow visualization of the entire skeleton.
Computed tomography (CT) can help with discrepancies not visualized on plain X-rays but picked up on bone scans. The thickness of cortical bone invasion, periosteal reaction, spatial structure and assessment of soft tissue metastatic extent can also be assessed on CT scans.
Magnetic resonance imaging (MRI) is very useful in assessing spine metastases and the extent of malignant spread into the spinal canal. Spinal cord compression, cauda equina compression, leptomeningeal spread, and intramedullary metastases to the spinal canal are best visualized on MRI.
Positron emission tomography (PET) combined with CT, used routinely for staging of certain cancers such as lung cancers and hematologic cancers can also detect the presence of malignant bone involvement.
Symptoms
Pain
The most common symptom for bone metastases is pain. However, why some bone metastases are painless while others are associated with pain is poorly understood. The presence of pain may not be correlated to the type of primary cancer, location, number or size of the bony metastases (3). The periosteum, which covers the external surface of bone, is highly innervated with nociceptors. It is believed that for some bone metastases, the nociceptors of the periosteum are activated causing the sensation of pain. It is also believed that chemical mediators of pain such as prostaglandins are important for the sensation of pain. Analgesics and radiotherapy for localized bone pain are standard therapies to treat symptomatic bone metastases.
Pathologic fracture
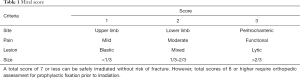
Bony metastases may weaken the integrity of normal bone such that the bone may be at risk of pathologic fracture. The Mirel score (4) is a scoring system which predicts the risk of pathologic fracture in patients with long bone metastases (Table 1). A total score of 7 or less can be safely irradiated without risk of fracture. However, total scores of 8 or higher require orthopedic assessment for prophylactic fixation prior to irradiation.
Full table
Spinal cord compression/cauda equina compression
Spine bone metastases may result in spinal cord compression, usually by extradural compression. Other types of spinal cord compression include intradural (intramedullary and leptomeningeal). Resulting symptoms include back pain, weakness, numbness, and sphincter deficits. This medical emergency requires urgent diagnosis and treatment to prevent permanent disability due to irreversible spinal cord injury. A population-based study reported that 2.5% of all cancer patients who died of their disease had at least one admission for malignant spinal cord compression (5). Management involves steroids (if not medically contraindicated), surgery for good prognosis patients who are medically and surgically operable, and radiotherapy (given post-operatively or in those who do not proceed to surgery) (6).
Cauda equina compression resulting from bone metastases compressing the spinal nerves (below the caudal end of the spinal cord) may lead to permanent weakness of legs and incontinence. Similar to spinal cord compression, therapy consists of steroids, surgery in selected patients and radiation.
Serum calcium disorders
Bone metastases disrupt the balance of osteoblastic and osteoclastic activity. In osteoblastic metastases, endothelial-1, platelet-derived growth factor, fibroblast growth factor, proteases and other factors lead to osteoblastic activity and formation of bone. Calcium is sequestered from the blood during the development of osteoblastic activity and hypocalcemia may arise (7-12). Treatment for hypocalcemia includes calcium and vitamin D supplementation.
In osteolytic metastases, factors such as parathyroid hormone-related protein, transforming growth factor beta, interleukin-1, interleukin-6 and tumour necrosis factor alpha lead to expression of RANK ligand and activated osteoclasts. Calcium is released and hypercalcemia of malignancy may occur (13-17). Treatment for hypercalcemia includes hydration and bisphosphonates.
Role for radiation (Table 2)

Full table
Pain
Overall pain relief with radiation ranges from 60–80% in the literature for the treatment of painful bony metastases and response occurs within 3 to 4 weeks of radiation (18-21). International consensus based on high quality randomized evidence reveals that a single fraction of 8 Gy is standard treatment for uncomplicated bony metastases causing pain (22). Uncomplicated bone metastases are painful bone metastases which are not associated with impending or existing pathologic fracture and are not associated with spinal cord or cauda equina compression. There is no benefit for pain relief in patients treated with higher total radiotherapy dose fractionation schemes for uncomplicated bone metastases. Single dose radiotherapy also reduces the burden of treatment visits for patients, is cost-effective (23,24) and is easy to implement in busy radiation oncology centres.
On the other hand, higher doses of radiotherapy may be considered for patients with extensive soft tissue involvement or to allow for remineralization for large osteolytic metastases (25). High dose radiation is also being used with stereotactic ablative body radiation for oligometastatic or oligoprogressive disease (26). Patients with oligometastatic disease (few sites of metastatic involvement) or oligoprogressive disease (few sites of metastatic site growth) may benefit from more aggressive local radiotherapy with the intent to improve local control, and possibly improve survival.
For patients who have recurrent pain after initial radiotherapy, approximately a third of patients will have pain improvement with repeat radiation (27).
Pain flare after radiotherapy is defined as a temporary increase in pain. The incidence of conventional external beam radiation induced pain flare ranges from 2–44% in the literature (28,29). Dexamethasone given with radiotherapy has been shown to reduce pain flare. A double-blind, randomized, placebo-controlled phase III trial involving 298 patients reported that two 4 mg dexamethasone tablets taken orally at least 1 hour before the start of a single 8 Gy radiation and then every day for 4 days after radiation, significantly reduced the incidence or pain flare. Twenty-six percent of patients randomly assigned to dexamethasone experienced pain flare compared to 35% in the placebo group, with a difference of 8.9% (95% lower confidence bound 0.0, one-sided P=0.05) (30).
Radiation for bone metastases may also cause radiation-induced nausea/vomiting. This can affect 50–80% of patients undergoing palliative radiation and is dependent on the normal tissues (e.g., stomach, bowel) receiving incidental radiation. Commonly, prophylactic anti-emetics are given prior to radiation for moderate to high risk radiotherapy volumes. Numerous anti-emetic guidelines are available which classify levels of emetogenic risk with radiation. An example of a moderate emetogenic risk site for radiotherapy is the upper abdomen. Low risk sites include the pelvis and thorax and a minimal risk site is the extremity. Generally, the guidelines recommend prophylactic serotonin receptor antagonists and optional dexamethasone for high risk and moderate risk sites. Prophylactic or rescue serotonin receptor antagonist, dopamine receptor antagonist or dexamethasone is suggested for low risk sites. Rescue serotonin receptor antagonist, dopamine receptor antagonist or dexamethasone is suggested for minimal risk sites (31).
Pathologic fracture
Patients with pathologic fracture or impending pathologic fracture should be assessed by orthopedic surgery. Surgery is used to stabilize bone, provide pain relief and to restore function. Postoperative radiation is generally recommended to reduce local progression and prevent prosthesis displacement (32).
Spinal cord compression/cauda equina compression
Patchell and colleagues reported a randomized trial in potentially operable patients with spinal cord compression treated with either surgery followed by radiotherapy or radiotherapy alone. Patients treated in the surgery group were more likely to walk after treatment (odds ratio 6.2; 95% CI, 2.0–19.8, P=0.001). Patients treated with surgery also were more likely to retain the ability to walk longer than those treated with radiotherapy alone (62% vs. 19%, P=0.01). The need for steroids and opioid analgesics were also significantly less in the surgery group (33).
Thus, in good prognosis patients who are medically and surgically operable, surgery for spinal cord compression should be considered especially if the spine is unstable or if there are bony fragments compressing the cord.
Another randomized trial for patients not proceeding to surgical stabilization was reported (34) comparing either a single 10 Gy of radiation versus 20 Gy in 5 fractions of daily radiation. There was no difference in overall mobility score change at 5 weeks. Quality of life scores were statistically improved after either radiotherapy regimen for the treatment of spinal cord compression. There was no statistically significant difference between a single 10 Gy radiation versus 20 Gy in 5 daily fractions on post treatment quality of life scores (35).
Rades and colleagues (36,37) reported that short course radiotherapy (4 Gy ×5) was not significantly inferior to 3 Gy ×10 in patients with malignant epidural spinal cord compression with respect to motor function, ambulatory status, local progression-free survival and overall survival. For patients with short life expectancy, Maranzano and colleagues (38) randomized 327 patients with malignant spinal cord compression to either 8 Gy ×2 versus a single 8 Gy radiation. No difference in response between the two radiation schedules was observed. Median overall survival was 4 months for both arms. The authors concluded that for patients with short life expectancy, a single dose of 8 Gy is just as effective and is associated with minimal toxicity and inconvenience as compared to 2 fractions of 8 Gy each. Thus, in poor prognosis patients with malignant spinal cord compression, a single dose of 8 Gy is preferred.
The use of stereotactic body radiotherapy for spine metastases (including spine metastases causing spinal cord compression) is being investigated by many groups (34,35,39,40).
Radiotherapy and molecular targeted therapy
There have been unforeseen and severe toxicities reported with molecular targeted agents and radiotherapy. One review on this topic (41) concluded that careful consideration is warranted when timing molecular targeted drugs with radiation, especially when used outside approved regimens or clinical trials (42). There have been a few published case reports of severe toxicity when certain molecular targeted agents were combined with palliative radiation. Examples include gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitors (43-45) and bronchial fistula associated with sunitinib in a patient previously treated with radiation therapy (46).
Limitations and future directions
The limitations of radiation for painful bony metastases include a 60–80% chance of pain relief. For some patients, pain will recur and although radiation may be repeated, the chance of pain response with repeat radiation may only be approximately 30%. In addition, the number of repeat radiotherapy courses which can be given is limited by potential toxicity of overdosing normal tissues in the radiation volume.
Newer radiation techniques such as stereotactic body radiation are being used for oligometastatic patients or oligoprogressive metastatic patients with the intent to improve local control and possibly survival. In addition, investigators are exploring the use of stereotactic body radiation for bone metastases in general. Whether these radiation approaches will benefit patients in terms of pain relief, quality of life, local control or survival as compared to conventional external beam radiotherapy will need to be confirmed in mature phase III trials.
Adjunctive therapies, such as analgesics, vertebroplasty, cementoplasty, bisphosphonates are also needed to help with pain control and quality of life. For prostate cancer patients, Radium-223 was associated with an overall survival advantage in castration-resistant prostate cancer (47). Whether radiopharmaceuticals will prove useful for other cancer primaries metastatic to bone will be determined with future trials.
Novel molecular targeted therapies may also prove useful in the future for the treatment of bone metastases.
Conclusions
Radiotherapy is an effective and standard modality for the treatment of complicated and uncomplicated bony metastases. Further strategies are needed to optimize pain relief, quality of life and survival in the bone metastases cancer population.
Acknowledgments
The authors thank Barb Zurowski for her administrative support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jan Gaertner, Charles B. Simone II and Fiona Lim) for the series “Clinical Challenges and Pitfalls in Early Palliative Care: Practical Knowledge and Guidance from other Medical Specialties” published in Annals of Palliative Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2019.07.09). The series “Clinical Challenges and Pitfalls in Early Palliative Care: Practical Knowledge and Guidance from other Medical Specialties” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [Crossref] [PubMed]
- Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol 1983;23:175-80. [Crossref] [PubMed]
- Hoskin PJ. Scientific and clinical aspects of radiotherapy in the relief of bone pain. Cancer Surv 1988;7:69-86. [PubMed]
- Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989.256-64. [PubMed]
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15:211-7. [Crossref] [PubMed]
- Loblaw DA, Mitera G, Ford M, et al. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys 2012;84:312-7. [Crossref] [PubMed]
- Body JJ, Niepel D, Tonini G. Hypercalcaemia and hypocalcaemia. Finding the balance. Support Care Cancer 2017;25:1639-49. [Crossref] [PubMed]
- Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol 2015;12:340-56. [Crossref] [PubMed]
- Canalis E, Centrella M, McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest 1988;81:1572-7. [Crossref] [PubMed]
- Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin- 1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med 1995;1:944-9. [Crossref] [PubMed]
- Yi B, Williams PJ, Niewolna M, et al. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res 2002;62:917-23. [PubMed]
- Schwartz GG. Prostate cancer, serum parathyroid hormone, and the progression of skeletal metastases. Cancer Epidemiol Biomarkers Prev 2008;17:478-83. [Crossref] [PubMed]
- Guise TA, Yin JJ, Taylor SD, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544-9. [Crossref] [PubMed]
- Powell GJ, Southby J, Danks JA, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res 1991;51:3059-61. [PubMed]
- Kakonen SM, Selander KS, Chirgwin JM, et al. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 2002;277:24571-8. [Crossref] [PubMed]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337-42. [Crossref] [PubMed]
- Dougall WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 2012;18:326-35. [Crossref] [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [Crossref] [PubMed]
- Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594-605. [Crossref] [PubMed]
- Sze WM, Shelley MD, Held I, et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy- a systematic review of randomized trials. Clin Oncol (R Coll Radiol) 2003;15:345-52. [Crossref] [PubMed]
- Falkmer U, Jarhult J, Wersall P, et al. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol 2003;42:620-33. [Crossref] [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- van den Hout WB, van der Linden YM, Steenland E, et al. Single versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003;95:222-9. [Crossref] [PubMed]
- Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol 2009;32:423-8. [Crossref] [PubMed]
- Chow E, Holden L, Rubenstein J, et al. Computed tomography (CT) evaluation of breast cancer patients with osteolytic bone metastases undergoing palliative radiotherapy-a feasibility study. Radiother Oncol 2004;70:291-4. [Crossref] [PubMed]
- Ning MS, Gomez DR, Heymach JV, et al. Stereotactic ablative body radiation for oligometastatic and oligoprogressive disease. Transl Lung Cancer Res 2019;8:97-106. [Crossref] [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomized, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [Crossref] [PubMed]
- McDonald R, Chow E, Rowbottom L, et al. Incidence of pain flare in radiation treatment of bone metastases: A literature review. J Bone Oncol 2014;3:84-9. [Crossref] [PubMed]
- Chiu N, Chiu L, Popovic M, et al. Latest advances in the management of radiation-induced pain flare, nausea and vomiting. Ann Palliat Med 2016;5:50-7. [PubMed]
- Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomized placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463-72. [Crossref] [PubMed]
- McKenzie E, Zaki P, Raman S, et al. Radiation-induced nausea and vomiting: a comparison between MASCC/ESMO, ASCO and NCCN antiemetic guidelines. Support Care Cancer 2019;27:783-91. [Crossref] [PubMed]
- Drost L, Ganesh V, Wan BA, et al. Efficacy of postoperative radiation treatment for bone metastases in the extremities. Radiother Oncol 2017;124:45-8. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Thirion P, O’Sullivan L, Clayton-Lea A, et al. ICORG 05-03: prospective randomized non-inferiority phase 3 trial comparing two radiation schedules in malignant spinal cord compression not proceeding to surgical decompression Int J Radiat Oncol 2014;90:1263-4. (abstract). [Crossref]
- Lee KA, Dunne M, Small C, et al. (ICLORG 05-03): Prospective randomized non-inferiority phase III trial comparing two radiation schedules in malignant spinal cord compression (not proceeding with surgical decompression); the quality of life analysis. Acta Oncol 2018;57:965-72. [Crossref] [PubMed]
- Rades D, Segedin B, Conde-Moreno AJ, et al. Radiotherapy with 4 Gy x5 versus 3 Gy x 10 for metastatic epidural spinal cord compression: Final results of the SCORE-2 Trial (ARO 2009/01). J Clin Oncol 2016;34:597-602. [Crossref] [PubMed]
- Rades D, Conde-Moreno AJ, Cacicedo J, et al. Comparison of two radiotherapy regimens for metastatic spinal cord compression: subgroup analyses from a randomized trial. Anticancer Res 2018;38:1009-15. [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [Crossref] [PubMed]
- Kim YJ, Kim JH, Kim K, et al. The feasibility of spinal stereotactic radiosurgery for spinal metastasis with epidural cord compression. Cancer Res Treat 2019;51:1324-35. [Crossref] [PubMed]
- Osborn VW, Lee A, Yamada Y. Stereotactic body radiation therapy for spinal malignancies. Technol Cancer Res Treat 2018;17:1533033818802304. [Crossref] [PubMed]
- Niyazi M, Maihoefer C, Krause M, et al. Radiotherapy and “new” drugs-new side effects? Radiat Oncol 2011;6:177. [Crossref] [PubMed]
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys 2016;95:632-46. [Crossref] [PubMed]
- Peters NA, Richel DJ, Verhoeff JJ, et al. Bowel perforation after radiotherapy in a patient receiving sorafenib. J Clin Oncol 2008;26:2405-6. [Crossref] [PubMed]
- Inoue T, Kinoshita H, Komai Y, et al. Two cases of gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitor for advanced renal cell carcinoma. World J Surg Oncol 2012;10:167. [Crossref] [PubMed]
- Hoshino Y, Hasegawa H, Ishii Y, et al. Two cases of bowel perforation associated with sunitinib treatment for renal cell carcinoma. Int J Clin Oncol 2012;17:412-6. [Crossref] [PubMed]
- Basille D, Andrejak M, Bentayeb H, et al. Bronchial fistula associated with sunitinib in a patient previously treated with radiation therapy. Ann Pharmacother 2010;44:383-6. [Crossref] [PubMed]
- Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomized trial. Lancet Oncol 2014;15:738-46. [Crossref] [PubMed]


