End-of-life discussions in practice: survey among Canadian radiation oncologists
Introduction
End-of-life (EOL) discussions improve patient satisfaction, quality and cost-effectiveness of care (1,2). Guidelines and educational objectives are available to help clinicians engage in quality EOL discussions (3,4). However, many radiation oncologists (ROs) are unlikely to discuss EOL issues until patients develop significant symptoms or families initiate the discussion (5,6). Previously described barriers among oncologists include limited knowledge or training, sense of failure in not being able to provide curative treatment, belief that patient’s outcome will be negatively impacted, uncertainty about the prognosis, ambiguity about the responsible physician, and lack of time.
ROs need to be proficient in discussing EOL issues. 40–50% of radiotherapy is given with palliative intent, and many patients in a RO practice have a short life-expectancy (7-9). Discussion of goals of care, patient values, and prognosis helps to direct care, especially in the setting of incurable or advanced cancers (6). At the end of their lives, many cancer patients encounter oncologists on a frequent basis; therefore, leaving EOL discussions to general practitioners may be inappropriate. Mack et al. showed that the majority of patients who died from cancer during a study period were cared for primarily by oncologists, but only 27% had an EOL discussion with their oncologist (10). In a survey performed in an inpatient unit, most patients preferred to discuss EOL issues with their oncologists rather than with their primary care physician (11).
With the passing of the bill C14 in Canada, and the legalization of Medical Assistance in Dying (MAiD), EOL care and decision making are becoming more complex. There is lack of clear consensus regarding how to approach MAiD requests among ROs.
To date, there are no publications describing whether EOL discussions take place specifically in the setting of radiation oncology practice in Canada. Given the volume of patients receiving palliative radiotherapy, it is important to understand the landscape of palliative discussions by ROs and the potential barriers. In addition, opinions of ROs regarding how EOL care discussions are changing as MAiD requests become commonplace in their practice is of great interest.
The objective of this study is to describe the frequency and content of EOL discussions among Canadian ROs and to identify barriers to these discussions.
Methods
Ethics approval was obtained from University of British Columbia (UBC) research ethics board (No. H18-00496), and informed consent was obtained from all of the responders. An email survey was sent to all board-certified ROs that are members of the Canadian Association of Radiation Oncology (CARO) (n=326). A second reminder request was sent 4 weeks later.
The survey (supplementary) consisted of 22 questions with 5 questions for basic demographic data and 17 questions soliciting practice pattern information and opinions regarding palliative care and MAiD. The secured Vancouver Coastal Health (VCH) Checkbox 6 program was used to create the questionnaire. The survey questions were devised to reflect previously identified important EOL topics for palliative patients (3,4). Some of the surveys from published studies were also referenced (6,12-14). The questions were reviewed by 4 independent investigators for suitability and face validity. They were designed to take only 10 minutes to complete. Two investigators were asked to comment on the survey regarding the content, clarity, and brevity. Once a consensus was reached, the survey was sent to the next investigator for comments and revisions. The same process was repeated until the fourth investigator agreed on the final revisions. The questionnaire covered topics such as experience with palliative care, EOL discussion patterns, MAiD, and suggestions for improvements. Responders were also asked to rank the perceived barriers to EOL discussions, and scores were tabulated based on ranking of the barriers. All responses were voluntary and anonymous.
Descriptive statistics and analytical statistics were performed on SPSS ver 22. Nonparametric Spearman’s correlations and Mann-Whitney U test were performed to assess association of basic demographic information and response outcomes. Kuskal-Wallis Test was performed to evaluate the association between self-reported amount of training in EOL discussion and the frequency, perception of, and confidence in such discussions.
Results
Demographic information is presented in Table 1. Sixty ROs responded out of 326 (19%). Average age was 45 (range: 32–76) years, average years in practice was 13 (range: 1–44), and 63.3% were male. The responses were represented by 7 provinces, with the highest representation from Ontario (30.0%). Most common specialty sites were GI (11.9%), GU (11.9%), lung (11.3%), head and neck (11.3%), and breast (10.1%). The least common specialty sites were hematologic (4.2%), skin (4.2%), sarcoma (2.4%), and pediatrics (1.8%). In terms of formal palliative care training, 16.0% had a rotation during medical school, 45.0% had a rotation during residency, 32.0% had 3 or more lectures. Only 5.0% had less than 3 lectures or no formal training.

Full table
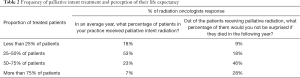
Over 50% of ROs reported that 25–50% of their practice consisted of palliative intent treatment, and 30% of ROs had greater than 50% palliative case load. Nearly 75% of ROs responded that more than 50% of the patients that receive palliative therapy would be expected to pass away within a year (Table 2).
Full table
Figure 1 shows RO survey response for frequency of EOL discussion topics. In a typical month of practice with patients starting palliative intent treatment, prognosis (57%) and goals of care (58%) were discussed more than 50% of the time. In contrast, advanced directive (40%) and planned site of death (12%) were discussed more than 50% of the time. There was no significant association between physician gender and the frequency of prognosis, site of death preference, advanced directive, and goals of care discussions (P=0.665, 0.513, 0.525, 0.268 respectively). There was no direct association between physician age and the frequency of EOL discussions (P=0.955, 0.800, 0.267, 0.482 respectively). However, there was significant association between the higher level of EOL discussion training and the frequency of the site of death preference discussion (P=0.041).
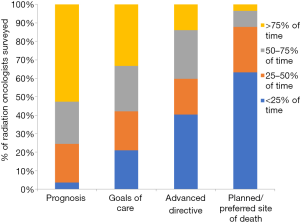
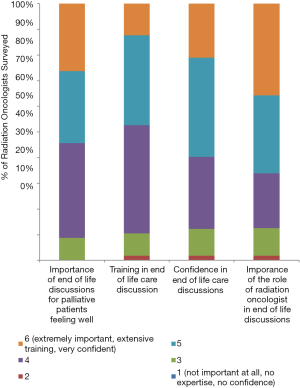
Figure 2 shows ROs opinion on aspects of EOL discussions. More than 90% of responders felt that the EOL discussions for palliative patients who were feeling otherwise well were important to very important. More than 60% of responders said role of RO is important in EOL discussion. Less than 50% of responders felt that they had adequate training in EOL discussions, and less than 60% said they were confident in engaging in the discussions. There were no significant association between gender and the rating of importance of palliative discussion in well patients, confidence in EOL discussion, importance of role of RO, or the amount of training in discussion (P=0.618, 0.182, 0.896, 0.080 respectively). There were no direct association between age and the above ratings (P=0.529, 0.234, 0.464, 0.444 respectively). There was significant association between the level of EOL discussion training and increased perception of the importance of RO role in EOL (P=0.006), importance of discussing EOL when the patient is otherwise well (P=0.039), and increased confidence in EOL discussion (P<0.01).
The most frequently identified barrier to EOL discussion was lack of time, followed by uncertainty about prognosis, concern for disappointment or hopelessness in patients, and uncertainty about the physician responsible for the discussion (Table 3). Other factors not listed in the questionnaire were hesitation to give too much information at once and appropriateness of the first consult as an opportunity to broach EOL discussion.
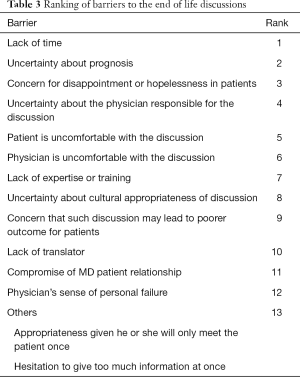
Full table
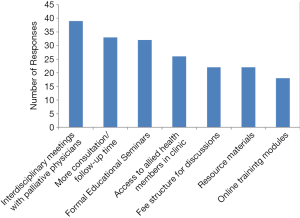
The most frequent preference for support to facilitate EOL discussion was interdisciplinary meetings with palliative care physicians (n=39), followed by more time for consultation/follow ups (n=33), formal educational seminars (n=32), access to allied health members such as general practitioners in oncology (GPO) or nurse practitioners (NP) (n=26), fee structure for EOL discussion (n=22), resource and reference materials (n=22), and online training module (n=18) (Figure 3).
MAiD was only routinely discussed by 3% of ROs. The majority (56%) of ROs said they provide information about MAID only when requested, 15% said they provide information about MAID on a case by case basis, 15% said they do not provide any information but refer to another colleague who is familiar with MAID process, and 5% said they do not talk about MAID at all.
Discussion
EOL discussions should occur with any patients facing serious and life-threatening illness, such as an incurable malignancy, or for any patients whose physicians would not be surprised if the patient died in the next year (4). This population consists of the majority of patients receiving palliative radiation therapy.
Strengths of this study are that it is the first survey of EOL care discussion practices among ROs in Canada. Participants represented a wide range of age, regions of practice, treatment sites, and radiation oncology residency rotation. In contrast, previous studies consisted of sampling of oncologists in confined regions, specialties, or treatment sites (13,14).
In our study, about 50% of ROs reported that they discuss prognosis more than 75% of the time that they evaluate patients for palliative intent treatment. This is consistent with previous literature which estimates about 50–70% rate of routine prognosis discussion with palliative patients (6,15). However, less than 10% of surveyed ROs discussed preferred or planned site of death routinely. This is unfortunately consistent with a study by Keating et al. that suggested the rate of discussion regarding hospice or site of death with terminally ill patients was low overall among medical practitioners, but it was significantly lower among ROs and surgeons compared to medical oncologists (5). Early discussion of site of death or hospice is recognized as an important part of EOL discussion (16,17). Many studies have suggested that patients prefer to die at home rather than in hospital, and hospice care at the EOL has been associated with better pain relief, quality of life, and potentially reduced cost of care (18-20). Such discussions occur too late in disease trajectory, and patients often miss the opportunity to die at home or at hospice as they wished (21-23). Therefore, exploring a patient’s preferred site of death or desire for hospice care should be an integral part of early EOL discussions.
Conversation about advance directive is also a crucial part of EOL discussion. In our study, less than 40% of ROs reported that they routinely discuss advance planning for patients undergoing palliative radiation. Advance directive reduces the cost of care and the frequency of aggressive treatments such as ventilation, intensive care admission, and resuscitation at the EOL (24,25). In turn, aggressive treatments are associated with worse patient quality of life and higher rate of major depressive disorder among the caregivers (24). Moreover, early advance care planning increases the likelihood that the patient wishes are known and followed (26,27). Recently, there have been many guidelines that advocate for routine advance directive discussion as part of quality oncology care, and advance directive should be part of routine EOL discussion (28,29).
Our study demonstrated that ROs recognize the importance of their role in early EOL discussion. More than 50% of the sampled oncologists felt that EOL discussion is important for palliative patients otherwise feeling well, and more than 60% felt that ROs are an important part of such discussions. However, less than 50% of the survey participants felt that they had enough training in it. Similarly, Tseng et al. found that ROs were somewhat or less confident in their ability to discuss prognosis and were less likely to engage in EOL discussion than medical oncologists (6).
In this study, ROs who reported more extensive EOL discussion training also had higher level of confidence in such discussion. They were also more likely to recognize the importance of engaging in EOL discussion earlier in the disease trajectory, planned site of death discussion, and RO’s role in EOL discussion. These results are not surprising, and more training will likely lead to better EOL discussions. Formal education in palliative care and EOL discussion is varied across training programs. In Canada, Royal College of Physician curriculum for radiation oncology program consists of only 1 month of palliative care (30). In the US, there is lack of formal instruction for palliative care as part of oncology curriculum (31,32). One solution may be an intense but short coaching for clinicians to better facilitate EOL discussion. A randomized trial by Bernacki et al. is currently enrolling clinicians to undergo 2.5-hour intense training to better utilize Serious Illness Conversation Guide (SICG) (33). Although results are not yet published, primary outcomes will be rate of goal-concordant care and patient peacefulness at EOL. Also, a study by Schenker et al. suggested that a 2-day intense training session led by a palliative care physician for oncology nurses was sufficient to improve symptom management and advanced planning for palliative patients (34). Another solution may be to increase formal palliative training during the residency, such as structured didactic teaching, utilization of standardized patients, formal feedback and evaluation sessions to increase resident comfort with routine EOL topics (32).
Other identified barriers to the EOL discussion were lack of time, uncertainty about prognosis, and concern for hopelessness and disappointment in patients. Similarly, a survey of Canadian medical oncologists identified lack of time, brief therapeutic relationship with patients, uncertainty in estimating prognosis, and desire to maintain hope as barriers in EOL (13). There may be several strategies to alleviate the time constraint for EOL discussions. One would be the use of educational tools, such as a pamphlet or a guidebook, in combination with clinician delegates for efficient discussion of EOL. Stein et al. demonstrated that for patients with metastatic cancer, the use of pamphlet containing information regarding communication and planning for future, as well as a psychologist to guide them through the decision making, resulted in earlier placement of advance directive, less likelihood of death in hospital, and less caregiver burden (35). Other studies demonstrated that the collaboration with an oncology nurse and the distribution of advance care guidebook to patients significantly increased the advance plan documentation in stage IV cancer patients (34,36). In addition, studies have shown that regular interdisciplinary palliative care meetings consisting of medical, radiation, surgical oncologists, as well as palliative physicians and nurses are feasible and may significantly improve quality of life, symptoms, and distress (37,38). It may be beneficial to have palliative care physician and a nurse at cancer-specific multidisciplinary rounds.
MAiD in Canada was first introduced in June 17th, 2016, after the passage of Bill C-14. Its implementation is in nascent stage, and the process requires fulfilment of stringent set of criteria. There have been mixed physician opinions about introduction of MAiD, reflecting the controversies regarding the logistics, ethics, and eligibility criteria surrounding it. Canadian Medical Association (CMA) survey in 2016 showed that 61% of physicians were unwilling to participate in MAiD, and 40% were not even comfortable referring the patient for MAiD (39). In contrast, among palliative physicians that were surveyed in 2017, 53% referred the patient for assessment for MAID, 75% provided information about MAID, and 80% explored the request themselves (40). Our study shows that ROs are very likely to either provide information about MAiD or refer to someone who is more knowledgeable about MAiD upon request. Although the survey cannot assess detailed opinions about MAiD from RO perspective, it demonstrates overall support for MAiD among ROs when patients request it.
The study is limited by a potential response bias due to the voluntary nature of the survey. However, if most of the study responders consisted of ROs actively engaging in EOL discussions already due to the response bias, then it would further strengthen the need for improvement in EOL discussion among all ROs. Also, the overall response rate was low at 19% and represents a small survey sample size compared to the total number of practicing ROs. This is an expected finding, as response rates of previous electronic surveys to ROs eliciting the supportive care practice patterns have been consistently below 20% (41,42). Many factors may account for the low response rate, such as lack of renumeration offer, selective interest in palliative care topics, and survey fatigue. Generalizability may be limited as regions of practice were not proportionally sampled. Yet, given the uniformity of radiation oncology training across Canada, trend would be expected to be similar across the provinces.
Conclusions
Canadian ROs recognize the importance of their role in EOL care discussion. While prognosis and goals of care are discussed frequently, advanced directive and planned site of death are not routinely discussed. ROs who have more extensive EOL discussion training not only have more confidence in EOL discussion, but also engage in such discussions earlier and more frequently. Structured short clinical training in EOL discussion among ROs may increase the quality and frequency of EOL discussions. Effective delegation of roles and patient education tools may alleviate the time constraint. Currently, MAiD is supported but not routinely discussed by ROs.
Supplementary
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by University of British Columbia (UBC) research ethics board (No. H18-00496) and written informed consent was obtained from all patients.
References
- Covinsky KE, Fuller JD, Yaffe K, et al. Communication and decision-making in seriously ill patients: findings of the SUPPORT project. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc 2000;48:S187-93. [Crossref] [PubMed]
- Foley K. Dismantling the barriers: providing palliative and pain care. JAMA 2000;283:115. [Crossref] [PubMed]
- Robinson CA, Fyles G, McKenzie M. Oncologist Experience Implementing Goals of Care Discussions in Everyday Ambulatory Oncology Practice: Implications for Education. J Cancer Educ 2017;32:301-7. [Crossref] [PubMed]
- Bernacki RE, Block SD. American College of Physicians High Value Care Task F. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med 2014;174:1994-2003. [Crossref] [PubMed]
- Keating NL, Landrum MB, Rogers SO Jr, et al. Physician factors associated with discussions about end-of-life care. Cancer 2010;116:998-1006. [Crossref] [PubMed]
- Tseng YD, Krishnan MS, Sullivan AJ, et al. How radiation oncologists evaluate and incorporate life expectancy estimates into the treatment of palliative cancer patients: a survey-based study. Int J Radiat Oncol Biol Phys 2013;87:471-8. [Crossref] [PubMed]
- Toole M, Lutz S, Johnstone PA. Radiation oncology quality: aggressiveness of cancer care near the end of life. J Am Coll Radiol 2012;9:199-202. [Crossref] [PubMed]
- Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer 1997;21:129-83. [Crossref] [PubMed]
- Barnes EA, Palmer JL, Bruera E. Prevalence of symptom control and palliative care abstracts presented at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys 2002;54:211-4. [Crossref] [PubMed]
- Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med 2012;156:204-10. [Crossref] [PubMed]
- Dow LA, Matsuyama RK, Ramakrishnan V, et al. Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol 2010;28:299-304. [Crossref] [PubMed]
- Rao AD, Chen Q, Ermoian RP, et al. Practice patterns of palliative radiation therapy in pediatric oncology patients in an international pediatric research consortium. Pediatr Blood Cancer 2017. [Crossref] [PubMed]
- Ethier JL, Paramsothy T, You JJ, et al. Perceived Barriers to Goals of Care Discussions With Patients With Advanced Cancer and Their Families in the Ambulatory Setting: A Multicenter Survey of Oncologists. J Palliat Care 2018;33:125-42. [Crossref] [PubMed]
- Odejide OO, Cronin AM, Condron N, et al. Timeliness of End-of-Life Discussions for Blood Cancers: A National Survey of Hematologic Oncologists. JAMA Intern Med 2016;176:263-5. [Crossref] [PubMed]
- Huskamp HA, Keating NL, Malin JL, et al. Discussions with physicians about hospice among patients with metastatic lung cancer. Arch Intern Med 2009;169:954-62. [Crossref] [PubMed]
- Dans M, Smith T, Back A, et al. NCCN Guidelines Insights: Palliative Care, Version 2.2017. J Natl Compr Canc Netw 2017;15:989-97. [Crossref] [PubMed]
- Ferris B. A Model to Guide Hospice Palliative Care: Based on National Principles and Norms of Practice. Ottawa, ON: Canadian Hospice Palliative Care Association 2013.
- Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013;309:470-7. [Crossref] [PubMed]
- Amano K, Morita T, Tatara R, et al. Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med 2015;18:270-3. [Crossref] [PubMed]
- Cheng SY, Dy S, Hu WY, et al. Factors affecting the improvement of quality of dying of terminally ill patients with cancer through palliative care: a ten-year experience. J Palliat Med 2012;15:854-62. [Crossref] [PubMed]
- Aldridge MD, Canavan M, Cherlin E, et al. Has Hospice Use Changed? 2000-2010 Utilization Patterns. Med Care 2015;53:95-101. [Crossref] [PubMed]
- Aldridge MD, Epstein AJ, Brody AA, et al. Association between Hospice Spending on Patient Care and Rates of Hospitalization and Medicare Expenditures of Hospice Enrollees. J Palliat Med 2018;21:55-61. [Crossref] [PubMed]
- Wang SY, Aldridge MD, Canavan M, et al. Continuous Home Care Reduces Hospice Disenrollment and Hospitalization After Hospice Enrollment. J Pain Symptom Manage 2016;52:813-21. [Crossref] [PubMed]
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665-73. [Crossref] [PubMed]
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169:480-8. [Crossref] [PubMed]
- Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. [Crossref] [PubMed]
- Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med 2010;362:1211-8. [Crossref] [PubMed]
- Bickel KE, McNiff K, Buss MK, et al. Defining High-Quality Palliative Care in Oncology Practice: An American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine Guidance Statement. J Oncol Pract 2016;12:e828-38. [Crossref] [PubMed]
- Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:96-112. [Crossref] [PubMed]
- Specialty Training Requirements in Radiation Oncology The Royal College of Physicians and Surgeons of Canada. 2014. Available online: http://www.royalcollege.ca/cs/groups/public/documents/document/y2vk/mdaw/~edisp/tztest3rcpsced000691.pdf. 2018.
- Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol 2017;7:e439-48. [Crossref] [PubMed]
- Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys 2017;97:884-5. [Crossref] [PubMed]
- Bernacki R, Hutchings M, Vick J, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open 2015;5:e009032. [Crossref] [PubMed]
- Schenker Y, White D, Rosenzweig M, et al. Care management by oncology nurses to address palliative care needs: a pilot trial to assess feasibility, acceptability, and perceived effectiveness of the CONNECT intervention. J Palliat Med 2015;18:232-40. [Crossref] [PubMed]
- Stein RA, Sharpe L, Bell ML, et al. Randomized controlled trial of a structured intervention to facilitate end-of-life decision making in patients with advanced cancer. J Clin Oncol 2013;31:3403-10. [Crossref] [PubMed]
- Obel J, Brockstein B, Marschke M, et al. Outpatient advance care planning for patients with metastatic cancer: a pilot quality improvement initiative. J Palliat Med 2014;17:1231-7. [Crossref] [PubMed]
- Ferrell B, Sun V, Hurria A, et al. Interdisciplinary Palliative Care for Patients With Lung Cancer. J Pain Symptom Manage 2015;50:758-67. [Crossref] [PubMed]
- Rogers JG, Patel CB, Mentz RJ, et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol 2017;70:331-41. [Crossref] [PubMed]
- Results of the CMA Member Survey on Medical Assistance in Dying. 2016.
- CSPCP Member Survey Medical Assistance in Dying (MAiD). 2018.
- Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol 2017;7:113-9. [Crossref] [PubMed]
- Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys 2009;75:1501-10. [Crossref] [PubMed]