
Palliative radiation oncology in pediatric patients
Introduction
Palliation of cancer-related symptoms is a fundamental aspect of clinical oncology. Children and adolescents with cancer are a particularly vulnerable population and their suffering may go unrecognized by care providers unfamiliar with the unique nature of pediatric malignancies (1,2). The majority of pediatric patients who succumb to their malignancies will experience symptoms requiring palliation at some point during their treatment, and in some instances well before the end of life (3-7). An understanding of the indications, timing, potential toxicity, and expected benefits for palliative interventions is thus essential knowledge for providers caring for pediatric cancer patients.
Pediatric palliative care is a broad discipline comprising all physical and psychosocial interventions intended to alleviate suffering and improve quality of life of children and their families (8). In the oncology setting, radiation therapy (RT) can be a valuable component of a multispecialty approach to palliation of pain or other symptoms arising in primary or metastatic cancers across a variety of organ systems. Palliative RT delivers radiation to symptomatic sites of disease or areas at high risk for becoming symptomatic, with the goal of controlling or reducing tumor burden. In contrast to RT given as part of definitive oncologic management, which may involve treatments delivered to a large area over the course of weeks to months, palliative RT courses are typically more limited in field extent, cumulative dose, and duration.
Palliative RT has been found to be effective in addressing symptoms arising from primary or metastatic brain tumors (9-11), painful bone metastases (12-14), spinal cord compression (15,16), airway or vascular compression (17-19), and bleeding (20-22), among other indications. Although RT is most frequently employed for the management of solid tumors across a variety of body sites, it has also been shown to be useful for the palliation of symptoms secondary to advanced hematologic cancers (23,24).
Pediatric malignancies represent a small subset of all new cancer diagnoses. It is therefore unsurprising that the overwhelming majority of studies evaluating palliative radiotherapy have been conducted in adult patient populations. Perhaps for this reason, the integration of palliative RT into the pediatric oncology setting has historically lagged behind utilization in adult oncology (25,26). While studies have shown that up to half of all RT treatments in adults are palliative in intent (27), a recent analysis of practice patterns across pediatric oncology centers found that palliative RT represented only 11% of all radiation treatments in children and adolescents (28).
Given this comparative paucity of treatments and data in children, interest and research into the use of pediatric palliative RT has increased substantially in recent years. When given appropriately, palliative RT can offer a meaningful opportunity to improve quality of life in this challenging group of patients. In this review, we present published data and provide a practical overview for pediatric oncologists, radiation oncologists, and other members of the care team regarding indications for RT as well as general considerations for fractionation, dosing, planning, and management of toxicities.
Symptoms in pediatric cancers
Childhood cancer encompasses a wide spectrum of histologies including leukemias, lymphomas, primary tumors of the brain and central nervous system, bone and soft-tissue sarcomas, and embryonal solid tumors such as Wilms tumor and neuroblastoma, among many other rare malignancies. Outcomes in children with cancer have improved dramatically in recent decades owing to advances in diagnosis and treatment (29), but some patients will still succumb to their disease. Among patients 0–14 years old, the National Cancer Institute (NCI) estimates a total of 11,060 new cancer diagnoses in the United States in 2019, resulting in 1,190 cancer-related deaths in children (30). Cancer continues to represent the leading non-accidental cause of death in the 1–14 age group (31).
Despite the decrease in death from malignancy, it has been increasingly well recognized that children and adolescents approaching the end of life as a result of their cancers have a diverse and often significant symptom burden. Retrospective studies in the United States and Netherlands using questionnaires for parents of children who recently died of their malignancies have attempted to quantify these symptoms (32,33). The most frequently reported in each study were pain, fatigue, and anorexia, with 70% or more of parents reporting each of these symptoms. Children also experienced dyspnea, nausea, bleeding, and seizures during the palliative phase of their care. Symptoms were rarely observed in isolation, reflecting the often multisystemic nature of end-stage malignancy.
Both local tumor progression and metastases to organs, soft tissue and bones can lead to significant physical and psychosocial distress. Growth of tumors in the abdomen and pelvis may interfere with normal bowel or bladder function, limit ability to tolerate oral intake, or cause vaginal bleeding in females. Disease in the neck and mediastinum can result in progressive dyspnea from obstruction of the trachea or proximal bronchial tree, superior vena cava syndrome, or dysphagia from esophageal compression. Intracranial tumors may cause a variety of symptoms depending on their location, including headache, seizures, nausea and vomiting, dizziness or ataxia, cranial nerve palsies, or various sensorimotor deficits. Pain is a potential symptom for nearly all sites.
Basics of palliative radiotherapy
Palliative RT delivers high-energy X-rays, electrons, or atomic particles to symptomatic primary tumors or metastases, inducing death of replicating tumor cells by accumulation of DNA damage. Palliative RT is provided with one radiation treatment (or fraction) given daily over a set number of days. Each fraction of RT usually requires the patient to lie still on a treatment table for approximately 10–15 minutes while radiation is delivered from a linear accelerator. Very young children or those unable to stay adequately still may require treatment under anesthesia.
Palliative RT doses are typically lower than those employed for definitive management of malignancies, decreasing the risk of acute side effects from damage to adjacent healthy tissue. While late radiation-induced toxicities are a justifiable concern with definitive RT in children and adolescents, they should not be a barrier in the palliative setting owing to the generally limited life expectancy of children receiving palliative RT. For example, secondary malignancies are exceedingly unlikely in the first 5 to 7 years following RT. The majority of palliative regimens are completed in 10 or fewer fractions, or two weeks of daily treatments.
Palliative RT can effectively supplement multimodal analgesia for pain control, antiemetic therapy, palliative chemotherapy, and other quality of life measures. RT can often be delivered concomitantly with additional interventions, including some chemotherapies, to decrease length of stay in hospitals or other inpatient facilities and minimize the time spent by children away from home.
Indications for palliative radiotherapy and expected outcomes of treatment
Extensive evidence exists for the utility of palliative RT in improving or controlling symptoms in adult patients with advanced cancer. While these data have frequently been extrapolated to the pediatric setting, numerous differences exist in the biology of childhood and adult cancers. Pediatric tumor histologies are generally more radiosensitive than adult malignancies (34), suggesting that responses to palliative RT may be more robust in children. Palliative response rates for selected histologies may be found in Table 1.
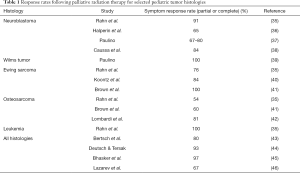
Full table
There is an emerging body of literature investigating symptomatic responses to palliative radiation in pediatrics. Similar to the adult setting, RT appears to be effective in palliating common cancer-related symptoms in children and adolescents, including bone pain, seizures or cranial neuropathies from intracranial progression, bleeding, and spinal cord compression.
Bertsch and colleagues assessed 90 children treated to 91 sites with urgent or emergent palliative RT, representing a diverse group of tumor pathologies and anatomic sites (43). Overall clinical response rate (improvement or stabilization) was 80% following palliative RT: 93% for bone or soft tissue pain, 85% for symptomatic spinal cord compression, 82% for intracranial neurologic compromise, 72% for respiratory compromise, and 66% for abdominal problems. Deutsch and Tersak reported similarly excellent control of painful bone metastases with palliative RT in patients under 19 years old (44); 55/59 of evaluable treatment courses (93%) demonstrated symptomatic improvement. Seventy-five percent of these treatments consisted of ≤5 fractions of RT, with over a quarter treated in only a single fraction, recapitulating data in the adult setting showing effective pain palliation with abbreviated radiation courses. Bhasker similarly showed a 97.5% symptom response rate (60% good or complete response) using 1, 5, or 10 fraction RT schedules for palliative RT in pediatric patients (45).
In Rahn et al.’s review of 83 palliative RT courses in 45 children, overall symptom response rate was 72% (35). When stratified by pathology, clinical responses to palliative RT were greatest for leukemia (100%), neuroblastoma (91%), and Ewing sarcoma (76%); osteosarcoma and primary CNS tumors were less likely to respond to treatment, with respective rates of 54% and 50%. Median survival after palliative RT in this cohort was 6.5 months. These histologic outcomes corroborate observations from prior studies demonstrating favorable responses to palliative RT in pediatric patients with metastatic or recurrent Ewing sarcoma (40,41) and neuroblastoma (36-38). Metastatic Wilms tumor has also been found to have high response rates to palliative RT (39).
Less radio-responsive tumors including osteosarcoma may still respond well to higher fractional doses. Lombardi investigated a regimen of 36 Gy given in 6 fractions over 2 weeks for local or distant palliation and found response rates of 81% with RT alone and 92% when RT was combined with chemotherapy (42). Lazarev et al. reported largely favorable outcomes in a single-institution analysis of hypofractionated palliative RT regimens with doses up to 10 Gy/fraction, with 1- and 2-year local control rates of 74% and 68%, respectively (46). Grade 3 or higher toxicities in this analysis remained low at 6.7%, with no treatment-related mortality.
Children and adolescents with cancer typically have fewer comorbid conditions than adults with cancer. Given that symptom improvement or resolution with RT may take days to weeks (47), care must still be taken to select patients most likely to derive benefit from palliative RT. Varma et al. compared clinical outcomes in children who received palliative RT either prior to or within the last 30 days of life (48). Symptomatic response rates were 89% when RT was given prior to this period but decreased markedly to only 28% for children who died within 30 days of treatment. Data suggest that futile treatment at the end of life is relatively uncommon for children, however. Median survival following initiation of RT in the aforementioned study was 124 days (48). Further, a retrospective analysis of patients <21 years old treated with RT at Indiana University and the University of Miami found that only 11/697 (1.6%) died within 30 days of RT or were unable to complete their treatment (49).
It is important for providers to clarify the intent of treatment to patients and their families. Lee et al. prospectively evaluated parental expectations following delivery of palliative RT (50). Though parents had been counseled on the indications and likely outcomes of treatment prior to RT, 76% expected prolonged survival and 40% expected their child to be cured. This parallels earlier work by Bluebond-Langner and colleagues suggesting that parents of children with cancer have complex responses to oncologist recommendations and the distinction between definitive management and symptom-directed care becomes frequently blurred (51).
Re-irradiation of pediatric brain tumors
The use of palliative RT in the setting of previously irradiated pediatric brain tumors represents a unique consideration. Recent studies support the use of repeat radiation in the setting of progressive brainstem gliomas, including diffuse intrinsic pontine glioma (DIPG) or diffuse midline glioma (DMG) (52). Wolff and colleagues conducted a retrospective analysis of individual treatments for pediatric DIPG that had progressed following definitive RT, primarily comprising systemic regimens but including seven children treated with repeat irradiation (53). Partial response was observed in 4/7 patients, exceeding the clinical response rate for any other modality, with a median post-RT event-free survival of nearly four months. Freese et al. reported on three additional cases of palliative RT to 20 Gy in 10 fractions for progressive DIPG, noting transient symptom improvement in two of the three patients treated, with no treatment-related toxicity (54).
In addition to symptomatic palliation, re-irradiation for brainstem gliomas may improve survival. Janssens et al. reported a case-control cohort study with 70 children aged 2–16 with progressive DIPG following primary RT, 31 of whom received palliative RT (55). The patients were matched by time to first progression to partially control for selection and other biases. Median overall survival was significantly improved at 13.7 months for those who underwent RT compared to 10.7 months for those who received chemotherapy or best supportive care. Lassaletta et al. also demonstrated that palliative reirradiation for progressive DIPG improved time from initial progression to death as compared to a historical, non-re-irradiated cohort (218 versus 92 days), with symptomatic improvement in 13/16 patients (56).
These studies (summarized in Table 2) support the overall safety and utility of moderately dosed courses of palliative RT for incurable, devastating central nervous system malignancies such as DIPG. It remains important to counsel patients and parents that while repeat irradiation may be beneficial, it is not intended or expected to be curative.

Full table
Potential barriers to palliative radiotherapy
Use of palliative radiotherapy in children and adolescents lags behind that for the majority of adult cancers. The reasons for this are complex and likely represent a combination of care provider perceptions, parental preferences, and logistical difficulties in accessing radiotherapy.
With regard to beliefs of pediatric care providers, studies have described diverse physician perceptions: that involvement of palliative care represents a failure in delivering definitive treatment (57), a perhaps too optimistic view that cure is possible even in the very late stages of disease (58), and the emotional toll of discussing and initiating a shift toward end-of-life care (59). Pediatric care providers also may not recommend palliative RT due to an incomplete understanding of its indications and potential benefits in children and adolescents with advanced cancers.
Logistical barriers may also play a role in the relative under-utilization of palliative RT in children and adolescents. Although some children’s hospitals have on-site radiation treatment facilities, this is not universally true. Off-site treatment requires coordinating daily transport, sometimes in a patient who may be symptomatic or require advanced monitoring and support staff. In radiation clinics that do not routinely treat pediatric patients, radiation oncologists may be less comfortable with caring for children and adolescents and providing management of side effects and other issues while undergoing palliative RT.
Daily anesthesia can also be a logistical challenge. While rarely needed for palliative RT in adults, anesthesia is not uncommon in pediatric RT, particularly for very young patients. Anesthesia was used in 38/365 (10.4%) cases of palliative RT across a large pediatric consortium, the majority for patients <5 years old (28); Mak et al. reported use of general anesthesia in 27/76 (36%) pediatric palliative RT courses (60). To our knowledge no studies have directly examined the impact of an anesthesia requirement on utilization of palliative RT in children. Given that daily sedation is resource-intensive and requires trained anesthesiologists or intensivists with particular expertise, their lack of availability in certain centers is likely a barrier to palliative pediatric RT.
Management of radiation side effects
Cumulative radiation doses for symptomatic palliation are typically less than those used in definitive treatment. Nevertheless, acute treatment-related toxicities remain a possibility for children and adolescents undergoing palliative RT. Similar to radiography, clinical radiation delivery is inherently painless. Discomfort or anxiety can still arise during the RT treatment process owing to the practical necessity of positioning and immobilization on the treatment table. For patients too young or unable to stay still, radiation treatments can also be performed under general anesthesia.
Nausea is a common side effect of palliative RT in which radiation beams pass through the stomach or bowel, and can also be seen with intracranial RT. Nausea can present after even a single fraction of radiation. In addition to treatment of the abdominal or pelvic viscera, sites prone to nausea during palliative RT include the lower thoracic and lumbar spine, sacrum, bony pelvis, and lung bases. Routine administration of antiemetics prior to daily treatments can mitigate RT-induced nausea but may need to be continued for several days after the completion of RT to allow for resolution. Nausea secondary to intracranial RT and refractory to antiemetics may require management with corticosteroids such as dexamethasone. Loose stools can similarly be seen with bowel radiation and can be managed with anti-diarrheals when bothersome.
For palliative radiation treatments targeting sites in the mediastinum, upper thoracic and cervical spine, or neck, RT-induced esophagitis is possible and can result in throat pain, dysphagia, or odynophagia. Esophagitis is typically not symptomatic below cumulative RT doses of 30 Gy; it is therefore less common at palliative dosing and then only at the conclusion of RT or days immediately following RT. If esophagitis occurs, local analgesics and/or pain medications including opioids may be necessary to reduce discomfort and encourage oral intake. These may be preferable to narcotic agents owing to their short duration of action and lack of systemic effects.
Conclusions
RT is an effective means of palliation for many of the most common symptoms that arise in children and adolescents with cancer. While numerous studies have validated the effectiveness of palliative RT in the adult oncology population, there is now a growing body of literature demonstrating similarly favorable outcomes in the pediatric population. The side effects of palliative RT in children are generally mild and manageable in both the inpatient and outpatient setting. Because effective palliation can occasionally be achieved with as little as a single radiation treatment, the judicious use of palliative RT may minimize the need for narcotic medications, additional time spent in the hospital, more invasive interventions, and disruptions to a child’s normal routine. These potential benefits make palliative RT an indispensable addition to a comprehensive palliative care effort for the pediatric cancer patient.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol 2008;26:1717-23. [Crossref] [PubMed]
- Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer 2011;117:2289-94. [Crossref] [PubMed]
- Sirkiä K, Saarinen UM, Ahlgren B, et al. Terminal care of the child with cancer at home. Acta Paediatrica 1997;86:1125-30. [Crossref] [PubMed]
- Drake R, Frost J, Collins JJ. The symptoms of dying children. J Pain Symptom Manage 2003;26:594-603. [Crossref] [PubMed]
- Bradshaw G, Hinds PS, Lensing S, et al. Cancer-related deaths in children and adolescents. J Palliat Med 2005;8:86-95. [Crossref] [PubMed]
- Jalmsell L, Kreicbergs U, Onelov E, et al. Symptoms affecting children with malignancies during the last month of life: A nationwide follow-up. Pediatrics 2006;117:1314-20. [Crossref] [PubMed]
- Goldman A, Hewitt M, Collins GS, et al. Symptoms in children/young people with progressive malignant disease: United Kingdom Children's Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics 2006;117:e1179-86. [Crossref] [PubMed]
- American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics 2000;106:351-7. [PubMed]
- Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [Crossref] [PubMed]
- Brown PD, Jackle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [Crossref] [PubMed]
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804. [Crossref] [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [Crossref] [PubMed]
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:4-12. [Crossref] [PubMed]
- Maranzano E., Trippa F, Casale M. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [Crossref] [PubMed]
- Rades D, Lange M, Veninga T. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524-30. [Crossref] [PubMed]
- Slawson RG, Scott RM. Radiation therapy in bronchogenic carcinoma. Radiology 1979;132:175-6. [Crossref] [PubMed]
- Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. [Crossref] [PubMed]
- Louie AV, Lane S, Palma DA, et al. Radiotherapy for intubated patients with malignant airway obstruction: futile or facilitating extubation? J Thorac Oncol 2013;8:1365-70. [Crossref] [PubMed]
- Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial BA09. Int J Radiat Oncol Biol Phys 2000;47:379-88. [Crossref] [PubMed]
- Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys 2001;49:107-16. [Crossref] [PubMed]
- Kim DH, Lee JH, Ki YK, et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J 2013;31:216-21. [Crossref] [PubMed]
- Jóhannsson J, Specht L, Mejer J, et al. Phase II study of palliative low-dose local radiotherapy in disseminated indolent non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Int J Radiat Oncol Biol Phys 2002;54:1466-70. [Crossref] [PubMed]
- Song JH, Son SH, Lee JH, et al. Defining the optimal dose of radiation in leukemic patients with extramedullary lesions. BMC Cancer 2011;11:428. [Crossref] [PubMed]
- Tucker TL, Samant RS, Fitzgibbon EJ. Knowledge and utilization of palliative radiotherapy by pediatric oncologists. Curr Oncol 2010;17:48-55. [Crossref] [PubMed]
- Weaver MS, Heinze KE, Kelly KP, et al. Palliative care as a standard of care in pediatric oncology. Pediatr Blood Cancer 2015;62 Suppl5:S829-33. [Crossref] [PubMed]
- Murphy JD, Nelson LM, Chang DT, et al. Patterns of care in palliative radiotherapy: A population-based study. J Oncol Pract 2013;9:e220-7. [Crossref] [PubMed]
- Rao AD, Chen Q, Ermoian RP, et al. Practice patterns of palliative radiation therapy in pediatric oncology patients in an international pediatric research consortium. Pediatr Blood Cancer 2017. [Crossref] [PubMed]
- Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for Children and Adolescents With Cancer: Challenges for the Twenty-First Century. J Clin Oncol 2010;28:2625-34. [Crossref] [PubMed]
- American Cancer Society. Facts & Figures 2019. American Cancer Society. Atlanta, GA, 2019.
- National Center for Health Statistics. National Vital Statistics Report. Centers for Disease Control and Prevention. Atlanta, GA, 2019.
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000;342:326-33. [Crossref] [PubMed]
- Theunissen JM, Hoogerbrugge PM, van Achterberg T, et al. Symptoms in the palliative phase of children with cancer. Pediatr Blood Cancer 2007;49:160-5. [Crossref] [PubMed]
- The Royal College of Radiologists. Good practice guide for paediatric radiotherapy, second edition. London: The Royal College of Radiologists, 2018.
- Rahn DA 3rd, Mundt AJ, Murphy JD, et al. Clinical outcomes of palliative radiation therapy for children. Pract Radiat Oncol 2015;5:183-7. [Crossref] [PubMed]
- Halperin EC, Cox EB. Radiation therapy in the management of neuroblastoma: The Duke University Medical Center experience 1967-1984. Int J Radiat Oncol Biol Phys 1986;12:1829-37. [Crossref] [PubMed]
- Paulino AC. Palliative Radiotherapy in Children with Neuroblastoma. Pediatr Hematol Oncol 2003;20:111-7. [Crossref] [PubMed]
- Caussa L, Hijal T, Michon J, et al. Role of Palliative Radiotherapy in the Management of Metastatic Pediatric Neuroblastoma: A Retrospective Single-Institution Study. Int J Radiat Oncol Biol Phys 2011;79:214-9. [Crossref] [PubMed]
- Paulino AC. Relapsed Wilms tumor: is there a role for radiation therapy? Am J Clin Oncol 2001;24:408-13. [Crossref] [PubMed]
- Koontz BF, Clough RW, Halperin EC. Palliative radiation therapy for metastatic Ewing sarcoma. Cancer 2006;106:1790-3. [Crossref] [PubMed]
- Brown LC, Lester RA, Grams MP, et al. Stereotactic Body Radiotherapy for Metastatic and Recurrent Ewing Sarcoma and Osteosarcoma. Sarcoma 2014;2014:418270. [Crossref] [PubMed]
- Lombardi F, Gandola L, Fossati-Bellani F, et al. Hypofractionated accelerated radiotherapy in osteogenic sarcoma. Int J Radiat Oncol Biol Phys 1992;24:761-5. [Crossref] [PubMed]
- Bertsch H, Rudoler S, Needle MN, et al. Emergent/urgent therapeutic irradiation in pediatric oncology: Patterns of presentation, treatment, and outcome. Med Pediatr Oncol 1998;30:101-5. [Crossref] [PubMed]
- Deutsch M, Tersak JM. Radiotherapy for symptomatic metastases to bone in children. Am J Clin Oncol 2004;27:128-31. [Crossref] [PubMed]
- Bhasker S, Bajpaj V, Turaka A. Palliative radiotherapy in paediatric malignancies. Singapore Med J 2008;49:998-1001. [PubMed]
- Lazarev S, Kushner BH, Wolden SL. Short Hypofractionated Radiation Therapy in Palliation of Pediatric Malignancies: Outcomes and Toxicities. Int J Radiat Oncol Biol Phys 2018. [Crossref] [PubMed]
- Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life. Cancer 2010;116:2716-25. [PubMed]
- Varma S, Friedman DL, Stavas MJ. The role of radiation therapy in palliative care of children with advanced cancer: Clinical outcomes and patterns of care. Pediatr Blood Cancer 2017. [Crossref] [PubMed]
- Panoff J, Simoneaux RV, Shah N, et al. Radiation therapy at end of life in children. J Palliat Med 2015;18:167-9. [Crossref] [PubMed]
- Lee BK, Apkon D, Wolfe J, et al. Palliative radiation therapy for pediatric patients: Parental perceptions. Int J Radiat Oncol Biol Phys 2017;99:S86. [Crossref]
- Bluebond-Langner M, Belasco JB, Goldman A, et al. Understanding parents' approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol 2007;25:2414-9. [Crossref] [PubMed]
- Ermoian R, MacDonald S, Laack NNI, et al. Reirradiation in Pediatric Patients With Recurrent Brain Tumors: A Last Hope, But One With Greatly Feared Consequences. Int J Radiat Oncol Biol Phys 2019;103:1-4. [Crossref] [PubMed]
- Wolff JE, Rytting ME, Vats TS, et al. Treatment of recurrent diffuse intrinsic pontine glioma: the MD Anderson Cancer Center experience. J Neurooncol 2012;106:391-7. [Crossref] [PubMed]
- Freese C, Takiar V, Fouladi M, et al. Radiation and subsequent reirradiation outcomes in the treatment of diffuse intrinsic pontine glioma and a systematic review of the reirradiation literature. Pract Radiat Oncol 2017;7:86-92. [Crossref] [PubMed]
- Janssens GO, Gandola L, Bolle S, et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 2017;73:38-47. [Crossref] [PubMed]
- Lassaletta A, Strother D, Laperriere N, et al. Reirradiation in patients with diffuse intrinsic pontine gliomas: The Canadian experience. Pediatr Blood Cancer 2018;65:e26988. [Crossref] [PubMed]
- Jackson VA, Mack J, Matsuyama R, et al. A qualitative study of oncologists’ approaches to end-of-life care. J Palliat Med 2008;11:893-906. [Crossref] [PubMed]
- Dalberg T, Jacob-Files E, Carney PA, et al. Pediatric oncology providers perceptions of barriers and facilitators to early integration of pediatric palliative care. Pediatr Blood Cancer 2013;60:1875-81. [Crossref] [PubMed]
- Szymczak JE, Schall T, Hill DL, et al. Pediatric Oncology Providers' Perceptions of a Palliative Care Service: The Influence of Emotional Esteem and Emotional Labor. J Pain Symptom Manage 2018;55:1260-8. [Crossref] [PubMed]
- Mak KS, Lee SW, Balboni TA, et al. Clinical outcomes and toxicity following palliative radiotherapy for childhood cancers. Pediatr Blood Cancer 2018. [Crossref] [PubMed]


