Early palliative care and quality of life of advanced cancer patients—a multicenter randomized clinical trial
Introduction
Patients with advanced cancer and their families frequently experience a high burden of suffering, which is not limited to the last days of life but occurs throughout the illness. The complex physical, psychological, social, and spiritual consequences of the disease typically affecting patients are often neglected by the common disease-centered approach (1,2). Palliative care (PC) is focused on patients and their families, with the aim to relieve suffering and achieve the best possible quality of life (QoL) (2). PC in oncology has traditionally been considered synonymous with end-of-life (EoL) care, to be administered when no cancer treatment is indicated (3,4). Based on evidence from randomized clinical trials (5-10) and meta-analyses (11,12), the oncology and PC communities (13-15) as well as the World Health Organization (WHO) (16) recommend early palliative care (EPC) soon after the diagnosis of advanced cancer. However, literature reviews emphasize that additional research is necessary to further understand the role of EPC in different cancer types and to characterize the optimal delivery and settings of the palliative interventions (11,17,18). At the time our study was designed, only two randomized trials assessing EPC interventions in patients with advanced cancer had been published (5,6). Bakitas et al. reported significantly higher QoL and mood for a psychoeducational intervention with nurse practitioners vs. SOC in patients newly diagnosed with different types of cancers (5). Temel et al. randomized patients with newly diagnosed metastatic palliative care non-small-cell lung cancer (NSCLC) to receive either EPC plus SOC or SOC alone, and observed that patients assigned to the intervention had significantly higher QoL scores, fewer depressive symptoms, less aggressive EoL care and longer survival (6). We thus report the results of a multicenter, randomized controlled trial of EPC versus SOC on the QoL of patients with different types of advanced cancers (NSCLC, pancreatic, gastric and biliary tract) treated at Italian cancer centers.
Methods
Study design
We conducted a pragmatic, multicenter, open-label, parallel randomized controlled trial, where enrolled patients were assigned to receive EPC integrated with SOC (intervention arm) or SOC alone (control arm). The trial, registered in the clinicaltrials.gov database (identifier: NCT02988635), was initiated in November 2014 and follow-up was completed in November 2016.
Participants
Consecutively enrolled patients were identified by research personnel. Eligibility criteria were: age 18 years or older; pathologically confirmed NSCLC, pancreatic, gastric or biliary tract cancer diagnosed within the previous 8 weeks; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0, 1, or 2; metastatic or locally advanced disease (but not susceptible to loco-regional treatments); life expectancy greater than three months; eligibility for first-line chemotherapy and/or biological therapy; completion of the QoL questionnaire ; provision of written informed consent at enrollment. Patients already receiving care from the PC service or previously treated with chemotherapy and/or biological therapy for advanced disease, as well as patients with NSCLC with EGFR mutation, were excluded. We decided to include patients with these cancer types because they share the same prognosis from the time advanced disease is diagnosed. Timing of enrollment after diagnosis of advanced disease was considered an eligibility criterion to define the earliness of the PC intervention, and ensure homogeneity of the study population with that of Temel et al. (6).
Setting
Patients were treated in outpatient and inpatient settings, at five Cancer Centers: three in University Hospitals (Parma, Bologna and Ferrara), and two in community hospitals (Piacenza and Fidenza). All centers are located in Emilia-Romagna, a Region in Northern Italy, and are European Society of Medical Oncology Designated Centers of Integrated Oncology and Palliative care (ESMO-DC-IOPC) (13).
Intervention
Table 1 shows how the intervention differs from standard care. Before study initiation, a meeting was held with the PC teams of participating centers to ensure uniformity. We did not conduct a pilot, exploratory study, because the palliative intervention was adapted from Temel et al. (6). This PC model is co-managed in a complementary manner by PC clinicians, attending to physical symptoms and psychosocial concerns, and by oncologists, focusing on cancer-specific treatments. Such coordination aims to meet supportive care needs, to ensure greater continuity of care and stave off unnecessary resource use (17). The core intervention was consultation and follow-up by the PC Team, composed of an oncologist specialized in PC and a nurse involved full time in PC. Patients met with the PC team within 2 weeks after enrollment, and at least every 2 weeks thereafter for 24 weeks. After protocol amendment, since August 2015, follow-up visits were scheduled every 3 weeks so that they would take place on the days chemotherapy or oncology consultations were scheduled. Additional visits with the PC team could be scheduled at the discretion of the patient, oncologist, or PC provider. General guidelines for the PC visits were adapted from the protocol of the Temel et al. study. The PC team documented provided care in the patient’s medical record. Physical and psychosocial symptoms were assessed using validated instruments, and the necessary interventions enacted according to individual patient needs. Patients assigned to standard care received anticancer and symptom control treatments provided by oncologists and nurses without formal PC training. They were offered no formal intervention, but PC referral was not denied, if requested. The patients in the control arm who were referred to the PC Team did not cross over to the EPC group or follow the specified PC protocol.
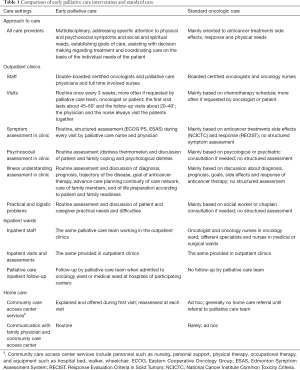
Full table
Outcome measures
The primary endpoint was the difference in the change of QoL, as measured from baseline (T0) to 12 weeks (T1), in the two groups. In the case of planned out-patient visits, follow-up could be performed within 3 weeks after that time point. Secondary endpoints were overall survival (OS), calculated from enrolment to 24 weeks or death (T2), and use of EOL care, defined as the percentage of deceased patients who in the 30 days preceding death: (I) received chemotherapy, (II) was admitted to hospital, (III) visited the emergency room. QoL was measured using the Functional Assessment of Cancer Therapy-General (FACT-G) scale (19), a 27-item internationally validated questionnaire divided into four primary HRQL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being. The total FACT-G score is the sum of the 4 subscale scores. The FACT-G was chosen following literature indications (20). The questionnaire was self-administered; upon patient request, assistance was provided by a nurse blinded to group assignment. In the case of incomplete responses, research staff provided help in filling in the questionnaire, and documented the reasons for the missing responses in a report form, thus limiting the number of missing data.
Patient socio-demographic and clinical characteristics were collected at baseline with an ad-hoc questionnaire and using medical records.
Sample size
To detect a significant between-group difference of at least 6.5 points in the change in the Fact-G score between T0 and T1, through a two-tail unpaired Student’s t-test with 80% power and 5% alpha, we estimated a sample size of 186 patients. This quota was increased by 30% (243 patients) based on the estimated number of deaths within 12 weeks (20%) and lost to follow-up (10%) at T1. For our estimation we used a MonteCarlo simulation (21). This approach enabled us to consider the heterogeneity of the different cancer types evaluated in this study. The following baseline values taken from Pearman et al. (22) were used: ECOG0 (µ=87.8, σ=14.2), ECOG1 (µ=78.8, σ=15.2), ECOG2 (µ=71.1, σ=−15.4).
Randomization and masking
Eligible patients were randomized before anticancer treatment to one of the two groups on a 1:1 allocation rate. To take into account center heterogeneity, stratified randomization was performed. Lists using a permuted block balanced procedure were generated for each participating center with the SAS v8 Statistical Software, and for each list a seed was defined. Lists were saved and implemented in a web-based e-CRF, to automatically assign the results of the randomization, thus ensuring allocation concealment.
Although complete masking of intervention was not feasible, blinding was ensured for health care staff in charge of data collection on QoL, which is very important since outcome measures involve some subjectivity.
Statistical analysis
Analyses were intention-to-treat. The primary endpoint was the comparison of the change in FACT-G score from enrollment to 12 weeks between study groups, assessed using the two-tail unpaired Student’s t-test. In addition, to complete case analysis, imputation method was used for missing observations as described in the FACIT Administration and Scoring Guidelines. An exploratory post hoc analysis was performed through multivariable linear regression model adjusted for QoL at baseline, group assignment, and controlled for other involved variables according to literature (age, PS, sex and died within 6 months) (23) or univariable analysis (P<0.2). A stepwise backward selection was applied to identify the most parsimonious model. All tests were two sided at a significance level of 0.05. No interim analysis was planned and no multiplicity test correction performed.
Statistical analyses were performed using R Statistical Software version3.1.3.
Results
Between December 2014 and May 2016, 281 patients were recruited, 139 in the control arm and 142 in the intervention arm. The participant flow diagram (Figure 1) shows that 63 of the 281 (22.4%) patients did not complete QoL at T1 because they were too ill (4 in the control arm) or had died (30 in the control arm and 29 in the intervention arm).
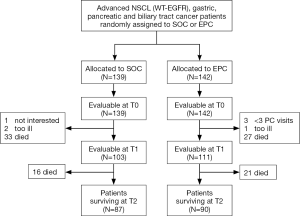
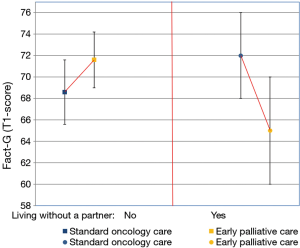
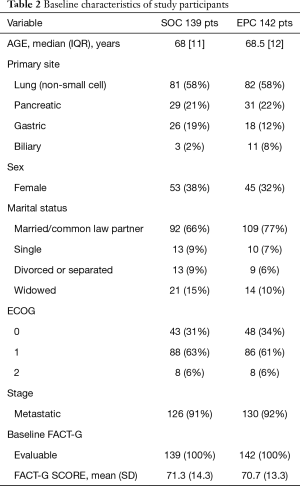
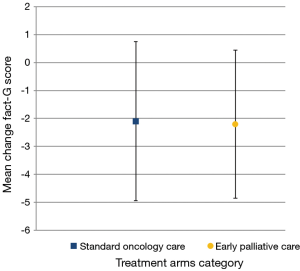
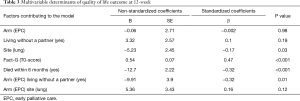
Baseline characteristics were well matched between the two arms (Table 2). Known prognostic factors, including age, sex, ECOG PS and presence of metastases were also balanced. Only a greater number of patients was observed with gastric cancer in the control arm and with biliary cancer in the intervention arm. At 24 weeks from enrolment, 63% of patients in both arms were alive (87/139 and 90/142, respectively). The median number of PC visits in the intervention arm was 8 (IQR, 5–10). Three patients had no PC visit, because of a rapid physical decline which did not allow them to come to the hospital. Mean values of FACT-G at T0 and T1 were for patients enrolled in the intervention arm, 72.3 (SD 12.6) and 70.1 (SD 15.5), vs. the control arm, 71.7 (SD 14.7) and 69.6 (SD 15.5), but the change scores (primary outcome) did not differ significantly between groups (Figure 2). Univariable linear regression models, adjusting for QoL at baseline and group assignment, identified the following variables related to QoL at T1 to be included in the multivariable analysis: ECOG (B=−17.24; P=0.08), Site-Lung (B=−2.76; P=0.13), Metastatic State (B=−6.39; P=0.043), Died within 6 Months (B=−12.21; P<0.001), the interaction of Living Without a Partner and Intervention Arm (B=−7.54; P=0.07). In the multivariable model, the following variables were statistically significant: Fact-G T0-score (B=0.44; P<0.001), Died within 6 months (B=−11.46; P<0.001), interaction of Living Without a Partner and the Intervention Arm (B=−8.73; P=0.03). Sex (B=−0.09; P=0.96), median age (B=−3.19; P=0.2), ECOG (B=−13.02; P=0.17), site lung (B=−4.63; P=0.07), metastatic status (B=−2.43; P=0.55) and their interaction with QoL at baseline or group assignment were not statistically significant. A backward stepwise selection process was used to build the most parsimonious model, expressed in terms of Akaike Criteria Information (AIC) index (from 1,097.76 to 1,089.03). In the final model identified in this way, the following variables were statistically significant: lung cancer (P=0.03), died within 6 months (P<0.001) and living without a partner and intervention arm (P=0.01), as shown in Table 3. The latter was particularly relevant (B=−9.91; P=0.01), as shown in Figure 3.
Full table
Full table
Discussion
This multicenter, randomized clinical trial failed to demonstrate that EPC, compared to SOC, ameliorates QoL after 3 months, measured with the FACT-G questionnaire, in advanced patients with different cancer types (lung, gastric, pancreatic, biliary tract). Several phase III, randomized clinical trials failed to demonstrate a benefit, in terms of 3-month QoL improvement, in patients with different types of advanced cancers receiving EPC (7,8,10,24-26). All these trials, like our study, evaluated patients with different tumors, but differed in the type of palliative intervention and in the QoL questionnaire used. In some of these trials, the EPC intervention was similar to that of our study and consisted in a consultation and follow-up by a PC team composed of a doctor and a nurse experienced in palliative care (7,10,26). In other studies, the EPC intervention consisted in structured PC telehealth nurse coaching sessions (8) or interventions by advanced practice nurses (24,25). The FACT-G QoL questionnaire, used in our study, was adopted in only two trials (10,25) whereas the majority employed other instruments such as FACIT-Sp (7), FACIT-Pal (8), McGill QOL (24) and QLQ-C30 scales (26).
Conversely, three randomized trials demonstrated that EPC is significantly better, compared to SOC alone, in improving QoL at 3 months (5,6,9). Only the trial of Bakitas et al. observed a QoL advantage for advanced patients with different cancer types (gastrointestinal, lung, genitourinary and breast). In this trial, patients were cared for in a rural setting by advanced practice nurses (5). The other two studies, unlike our trial, showed better QoL at 3 months only in a specific type of tumor, NSCLC and pancreatic advanced cancer, respectively; patients received the EPC intervention by palliative care physicians and advanced practice nurses (6,9). A recent Cochrane systematic review (11), pooling data from seven studies with 1,028 analyzed participants, showed that the patients with advanced cancer receiving EPC had significantly higher QoL than those receiving SOC. However, by conventional criteria, this effect is considered small and the results should be interpreted with caution, because of the very low to low certainty of current evidence and between-study differences regarding participant populations, interventions, and methods.
Several reasons could explain the negative findings of our study. One could be that the evaluation of QoL after only 3 months might not have allowed enough time to observe potential EPC benefits, as was highlighted in the cluster randomized trial of Zimmerman et al. (7) and in the randomized trials of Bakitas et al. (8) and Temel et al. (10), where improvements were detected starting from the fourth month or later. Furthermore, the heterogeneity of study population in terms of different tumor sites may have contributed to dilute the positive effect of EPC and could reflect a different response of a mixed population (NSCLC vs. gastrointestinal cancers). In fact, two of the three randomized trials with positive results at 3 months were restricted to only one cancer type (6,9). A third possible cause of effect dilution is the recruitment approach that did not include screening for palliative needs. In our study, a large proportion of patients exhibited a good PS (PS 0 34% in EPC and 31% in SOC arms) and very few patients had a poor PS (PS 5% in EPC and 6% in SOC arms). This may express a low symptom burden, which would make early positive changes in QoL difficult to obtain. Also, the high “core” in palliative care of oncologists of the ESMO Designated Centers participating in our study could be further cause of dilution effect. Finally, like other randomized clinical trials on EPC for different cancer types (7,10), we employed the “general” FACT (FACT-G) as measurement instrument rather than “tumor-specific” FACT (FACT-Lung, FACT-Hep). It is known that scales which produce a global QoL score may mask important differences between patients, and the negative findings obtained by trials on EPC may be due in part to the limitations in the assessment tools (27). On the other hand, the use of multiple questionnaires would have implied increased burden for patients, a larger sample size, more resources and longer study duration.
A post-hoc multivariable analysis performed in this trial highlighted some aspects which may guide future research on EPC aimed to provide more precise indications and strengthen recommendations on its use. Firstly, EPC appeared to be more beneficial in patients with lung cancer compared with other cancer types, as stated by Temel et al. (10); therefore, the assumption that the same model of care is equally effective across different diseases should be verified, as emphasized by Davis et al. (28). Secondly, we observed that the intervention was more effective for patients living with a partner. To our knowledge, this variable has not been explored by previous trials; this association seems plausible, since it may have led to better treatment compliance. This finding suggests that the integrated PC model may not be suitable for all patients, and that alternatives, such as community—and home-based palliative care services, may be more appropriate for certain populations (12,18,29). It also suggests that the models of care may depend on cultural background, family dynamics and patient location (10,28). Further research is therefore necessary to define the best models of care for diverse populations and disease types (12,28). Thirdly, our data suggest that in patients closest to death the intervention does not influence QoL. This seems not to be in line with the findings of the recent Temel et al. study (10), where patients assigned to EPC experienced better QoL 2 and 4 months before death.
This study has some important limitations. First, although we advised all centers to complete a predefined checklist on issues to be addressed during the PC consultation, we did not check to ensure that this had been done. Second, we did not study the palliative intervention in a pilot, exploratory, feasibility study. Third, patients and clinicians could not be blinded to group assignment. Blinding would have allowed for a more rigorous assessment. However, the assessors were blinded.
In conclusion, this study failed to demonstrate the superiority of EPC versus SOC in improving the 3-month QoL of different advanced cancer patients. However, this multicenter trial suggests that it is feasible to accrue, in a short period of time, a large number of incurable advanced cancer patients from community-based Cancer Centers, reflecting a real-word situation. In line with the most recent literature reviews and meta-analyses (11,12,28,30), we conclude that the mixed findings observed in randomized trials, and the low quality identified for many studies, call for further research in this field before generalizing results to practice. In particular, our findings suggest the need to carefully select patients according to symptom burden, prognostic and social factors, to target the intervention to those individuals who may benefit the most, as well as to determine the optimal timing to intervene and to assess outcomes.
Acknowledgments
We thank Francesca Diodati, affiliated with the University Hospital of Parma, Research and Innovation Unit, for her support with literature review and writing of the manuscript.
This work was supported by the Programma di ricerca Regione-Università, Regione Emilia-Romagna, bando “Ricerca per il Governo clinico” 2013. Project title “Valutazione dell›efficacia del modello assistenziale “Simultaneous Care” nel miglioramento della qualità di vita dei pazienti affetti da neoplasie maligne. Studio clinico controllato e randomizzato”. The funding source had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The trial was approved by the Parma University Hospital Ethics Committee (the coordinating center) (No. 8/14) and by the Ethics Committees of all participating Centers (HEC Piacenza No. 2014/0041111; HEC Bologna No. 2288/14).
References
- Harrison JD, Young JM, Price MA, et al. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009;17:1117-28. [Crossref] [PubMed]
- Glare PA. Early implementation of palliative care can improve patient outcomes. J Natl Compr Canc Netw 2013;11:S3-9. [Crossref] [PubMed]
- Meier DE, Brawley OW. Palliative care and the quality of life. J Clin Oncol 2011;29:2750-2. [Crossref] [PubMed]
- El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol 2011;9:87-94. [Crossref] [PubMed]
- Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. [Crossref] [PubMed]
- Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 2015;33:1438-45. [Crossref] [PubMed]
- Maltoni M, Scarpi E, Dall'Agata M, et al. Early Palliative Care Italian Study Group (EPCISG). Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016;65:61-8. [Crossref] [PubMed]
- Temel JS, Greer JA, El-Jawahri A, et al. Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol 2017;35:834-41. [Crossref] [PubMed]
- Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017;6:CD011129. [PubMed]
- Gaertner J, Siemens W, Meerpohl JJ, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357:j2925. [Crossref] [PubMed]
- Zagonel V, Cavanna L, Cetto G, et al. The medical oncologist's role in palliative care: AIOM's position. Tumori 2009;95:652-4. [Crossref] [PubMed]
- Cherny N, Catane R, Schrijvers D, et al. European Society for Medical Oncology (ESMO) Program for the integration of oncology and Palliative Care: a 5-year review of the Designated Centers' incentive program. Ann Oncol 2010;21:362-9. [Crossref] [PubMed]
- Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. [Crossref] [PubMed]
- WHO | WHO Definition of Palliative Care. 2017. Available online: http://who.int/cancer/palliative/definition/en/
- Howie L, Peppercorn J. Early palliative care in cancer treatment: rationale, evidence and clinical implications. Ther Adv Med Oncol 2013;5:318-23. [Crossref] [PubMed]
- Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349-63. [Crossref] [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [Crossref] [PubMed]
- Luckett T, King MT, Butow PN, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol 2011;22:2179-90. [Crossref] [PubMed]
- Landau S, Stahl D. Sample size and power calculations for medical studies by simulation when closed form expressions are not available. Stat Methods Med Res 2013;22:324-45. [Crossref] [PubMed]
- Pearman T, Yanez B, Peipert J, et al. Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer 2014;120:2902-9. [Crossref] [PubMed]
- Zimmermann C, Burman D, Swami N, et al. Determinants of quality of life in patients with advanced cancer. Support Care Cancer 2011;19:621-9. [Crossref] [PubMed]
- Tattersall MH, Martin A, Devine R, et al. Early contact with palliative care services: a randomized trial in patients with newly detected incurable metastatic cancer. Palliat Med Care 2014;4:170.
- McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med 2015;18:962-9. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Damkier A, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: The Danish Palliative Care Trial. Palliat Med 2017;31:814-24. [Crossref] [PubMed]
- Salisbury C, Bosanquet N, Wilkinson EK, et al. The impact of different models of specialist palliative care on patients' quality of life: a systematic literature review. Palliat Med 1999;13:3-17. [Crossref] [PubMed]
- Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015;4:99-121. [PubMed]
- Kamal AH, Currow DC, Ritchie CS, et al. Community-based palliative care: the natural evolution for palliative care delivery in the U.S. J Pain Symptom Manage 2013;46:254-64. [Crossref] [PubMed]
- Hui D, Arthur J, Dalal S, et al. Quality of the supportive and palliative oncology literature: a focused analysis on randomized controlled trials. Support Care Cancer 2012;20:1779-85. [Crossref] [PubMed]