
Palliative radiation oncology programs: design, build, succeed!
Introduction
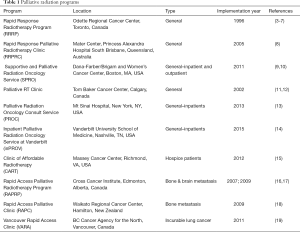
In many countries, access to radiotherapy centers is limited with wait times for consultations ranging from a few weeks to several months (1,2). Rapid access clinics arose in many centers to decrease the time interval from referral to the completion of treatment. See Table 1.
Full table
Often those patients with advanced cancer, who are dealing not only with the symptoms of advanced cancer but existential questions about life and what is most meaningful, are seen as inpatients or add-on patients (19). The nature of how these consults are facilitated—as an inpatient or add on to a full clinic schedule—does not always permit adequate time to attend fully to a patient’s spiritual needs, goals of care, and symptoms. This often means that full attention to these issues is deferred (20).
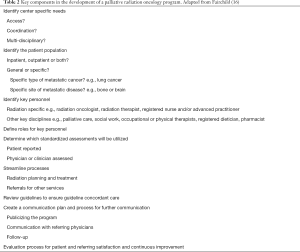
While rapid access clinics were formed as a reaction to prolonged wait times, they have also tried to attend to the more complex needs of patients with advanced disease, ensure guideline compliant care and enroll patients on clinical trials (4,16,17,21). Key to the development of a palliative radiation oncology program is to identify gaps between currently provided care and ideal or enhanced care. Once these gaps are identified, processes must be put in place to address each gap. Ideally, each opportunity has a metric that can be collected to measure the success of the clinic and identify other opportunities to improve or enhance care.
After the gaps and metrics are identified, successful implementation of a rapid access clinic identifies key personnel and assigns each role specific responsibilities that maximizes their scope of practice. The ideal rapid access clinics thoroughly pre-screen patients and coordinate the radiation simulation, planning and treatment delivery process to ensure consult, simulation and treatment on the same day. Typically, relatively simple radiation treatment plans, e.g., parallel opposed fields, and simplified dosimetry are utilized to facilitate rapid turnaround. This not only improves access, but timeliness and cost of care. In successful rapid access clinics, the time from referral to delivery of the first, and potentially only, fraction of radiation dramatically decreased. See Table 2.
Full table
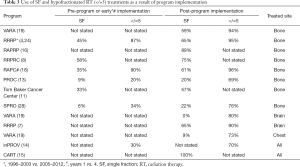
The standard of care for an uncomplicated bone metastasis is treatment with a single fraction (SF) of 8 Gy, but rates of SF treatment are still low (22-25). Clinics or services staffed by radiation oncologists with a specific interest and expertise in palliative care offer increased opportunity to provide guideline compliant care (26,27). Some patients are more appropriately treated with a small number of fractions of radiation therapy (RT) (hypofractionated RT). This is typically defined 5 or fewer fractions of radiation therapy. Successful rapid access clinics have demonstrated an increase in the number of SF and hypofractionated radiation therapy. See Table 3.
Full table
Patients with advanced cancer have unique needs given their generally higher symptom burden, mobility and comfort constraints and existential distress that comes from suffering and dealing with end of life issues. Development of specialized clinics with co-located services such as social work, specialized palliative care professionals, clinical pharmacists and others help address advanced directives, pain and symptom management, and psychosocial distress in a patient centric process (29). Pain management strategies are especially important considerations for comfort and reproducibility during the radiation therapy simulation and treatment process and in the interval until the palliative effect of radiation therapy is achieved, which can be as short as a few hours but may be as long as 3–4 weeks.
One key component of a palliative radiation oncology program is the use of standardized assessments to help assess patient symptoms and track outcomes. Multiple assessment tools exist; the choice of which assessment tool and whether it is patient reported or clinician reported will vary from program to program. Without the use of standardized tools, it is difficult to record program successes and address any deficiencies.
Rapid access programs
The first rapid access program was developed at Toronto-Sunnybrook Regional Cancer Center, now the Odette Regional Cancer Center, Toronto, Ontario by Dr. G. Thomas in 1995 with the goal of reducing wait times (5,30). The Rapid Response Radiotherapy Program (RRRP) started with a half-day clinic two times per week with the goal of providing a consultation within 3 days of the referral as well as same day consultation, simulation and start of radiation treatment. In the first year of operation, they saw a 16% increase in the number of administered palliative courses of radiation, compared with a 4% increase in the 3 years prior (30). In their first 8 years of operation, 3,290 patients were seen, and the volume of patients increased from about 200 to 500 patients per year (4). The majority of patients were referred for treatment of bone metastasis (70%) and brain metastasis (14%). Ninety percent of patients were seen within 2 weeks of the referral and 38% within 1 week. Eighty-five percent of patients had a simulation on the same day as the consultation and 60% started RT the same day, with a median time from referral to start of RT of 8 days (4,7). Due to the growth in the program, there are now 5 half-day clinics (7).
Similar general rapid access palliative radiation programs were developed at the Ottawa Regional Cancer Center, in Alberta at the Tom Baker Cancer Center, and at the Mater Center at Princess Alexandra Hospital in Australia (8,21,31). Specialized clinics have been developed in other centers for streamlined treatment of bone metastasis, brain metastasis and incurable lung cancer (16-19).
In the US, since access is less of an issue, streamlined, multidisciplinary palliative radiation oncology services have developed (9,13,14,32). The first U.S. palliative radiation oncology service was the result of a collaboration between the Dana-Farber/Brigham and Women’s Cancer Center’s (DFBWCC) Departments of Radiation Oncology and Psychosocial Oncology and Palliative Medicine (Boston, MA, USA). Named the DFBWCC Supportive and Palliative Radiation Oncology Service (SPRO), it offers a 24-hour clinical team to support inpatient and outpatient palliative radiation oncology needs via a single access phone number. Key components of SPRO include at least twice-daily inpatient rounds and triage of consults according to the clinical need. Since its inception, it has seen an increase in the number of weekly consults from 10 (pre-SPRO) to 16, about 60% of which are inpatient consultations (9). Similar palliative radiation oncology services have been developed at other institutions (13,14,32).
See Table 1 for a listing of Palliative Radiation Oncology Programs with references.
Improved outcomes with dedicated palliative radiation oncology programs
Access
Successful program implementation decreases the interval between referral and consultation and between referral and start of radiation therapy (4-6,8,18). While the metrics published vary from program to program, every program demonstrated decreases in the time from referral to consult and the time from consult to the start of radiation therapy. For example, in the RRRP, the percentage of patients seen in consultation within 7 days increased from 68% to 100% from 1999 to 2008 and the median number of days decreased from 8 to 3. With these programs, 60–72% receive treatment on the same day as the consult (7,18,19). The remaining patients require additional work-up or a more complicated treatment plan. Insurance prior authorization was not noted as a reason for delay in any of the publications but can certainly be a factor in the US.
As part of the pre-consult process, rapid access clinics pre-screen patients for the appropriateness of the referral, which ensures that most of the patients seen in the dedicated clinics have the appropriate work-up completed and indication for expedited radiation therapy. The clinics are set up with dedicated staff with clear role delineation, set simulation times, dosimetric and/or physics support. To simplify the radiation planning process, simple field arrangements and techniques are used, e.g., parallel opposed fields. Depending on the thoroughness of the pre-consultation process, patients may need additional imaging or work-up or they may prefer to come back another day. Given the design of these clinics, they are set-up to consult, simulate and treat a patient on the same day.
For patients with bone metastases, only one visit is required for the vast majority of patients who present with uncomplicated bone metastasis. In the Rapid Access Palliative Radiotherapy Program, (RAPRP, Cross Cancer Institute, Edmonton, Alberta, Canada) 89% of patients in the bone metastasis clinic received SF RT for uncomplicated bone metastasis. The remaining 11% of patients had a known indication for multi-fraction treatment.
Patients receiving hospice care have limited access to radiation therapy. The Clinic Offering Affordable Radiation Therapy (CART, Virginia Commonwealth University, Richmond, VA, USA) designed a 4-hour visit for pre-screened hospice patients to be assessed by a radiation oncologist, simulated for treatment, and receive a SF of radiation therapy (15). Simple field arrangements and rectangular fields obviated the need for a medical dosimetrist which helped minimize resources and keep costs and time down. Hospice referrals for radiation therapy increased from none to approximately one referral every 3 months. This design and selection process, ensures treatment on the same day as the consult for hospice patients (15).
Cost
Palliative radiation oncology inpatient services may decrease total hospital costs, median length of stay, and number of inpatient bed days (13,14). These consult services are staffed with radiation oncologists more attuned to the principles of palliative radiation, offering the shortest possible course of radiation to a patient to achieve the desired palliative effect (33). By using simpler techniques and shorter courses of radiation, these programs have the potential to decrease length of stay and cost. Similarly, these consult services incorporate palliative care services into the consultative process. Palliative care consults help the medical team evaluate and attend to a patient’s goals and spiritual needs and often avoid unwanted or unnecessary care. Access to palliative care in advanced cancer leads to less expensive care as patients opt not to receive more aggressive care that is not likely to benefit them and not concordant with their goals (34-36).
At Mt. Sinai Hospital, implementation of the Palliative Radiation Oncology Consult Service (PROC, New York, NY, USA) resulted in a decrease in the mean cost of hospitalization by approximately $20 K. Hospital stays decreased by an average of 8.5 days and there was an increase in the use of palliative care and shorter courses of radiation therapy. The median length of stay for patients receiving palliative radiation decreased from 21 to 9 days with implementation of the inpatient Palliative Radiation Oncology service at Vanderbilt (inPROV, Vanderbilt University, Nashville, TN, USA) (14). Annual inpatient days decreased from 742 to 337. Hypo-fractionated radiation courses increased from 30% to 70%.
Innovation
Traditional processes in a radiation oncology department involve a consultation with the radiation oncologist to determine whether there is a role for radiation therapy in the management of a patient’s cancer. If radiation therapy is appropriate and the work-up is complete enough for decision making, a patient is scheduled for a simulation. Historically, this is done in a separate simulation unit and often is a separate visit. Once a patient has been set-up into a position that allows reproducible access to the site being treated, treatment volumes are noted, normal structures are identified and a radiation plan that maximizes the dose of radiation to the target volume while delivering as little dose as possible to the surrounding normal organs is designed. This process can take anywhere from several hours to several days depending on the complexity of the patient’s case, the volume of patients, and the systems in place at a particular institution.
One of the ways patients being treated to palliate a symptom can be expedited is to use simple immobilization devices and simple (e.g., parallel opposed fields) beam arrangements. In addition, linear accelerator-based imaging tools can further streamline the radiation planning process. Patients can be simulated on the linear accelerator with standard immobilization devices, treatment can be planned with simple shielding and then treated within a span of about 20–30 minutes using either kV images or cone beam CT (37,38).
Increased use of single or shorter courses of radiation therapy (see Table 3)
Bone metastasis
In all rapid access or dedicated palliative radiation oncology programs with reported outcomes, the use of SF RT for bone metastasis increased after implementation of the program (see Table 3). For example, at the RRRP 1999–2005 SF use increased SF from 45% to 65% (4,6). In the RAPRP bone metastasis clinic 89% of patients were treated with a SF while the remaining 11% had a recognized indication for multi-fraction treatment (16).
In patients treated through the specialized palliative RT clinic at the Tom Baker Cancer Center between 2003–2005, 57% received a SF for their first course of RT for bone metastasis compared with 33% receiving usual care in the cancer center (11). In addition, more patients (41%) returned for palliative RT to the specialized palliative RT clinic when compared to those patients receiving usual care (29%). This is likely due to the expedited process of evaluation, simulation and treatment in their specialized rapid access clinic. There were similar rates of repeat irradiation to the same site (18%) for patients receiving usual care vs. in the specialized palliative RT clinic care.
At SPRO, rates of single and hypo-fractionated radiation therapy use for bone metastasis increased from 6% to 22% (SF) and from 28% to 54% (hypo-fractionated) (28).
Lung cancer
Practice patterns for palliation of intrathoracic symptoms of lung cancer have been more resistant to change and vary by hospital. Eleven randomized controlled trials of fractionation demonstrate the equivalence of shorter courses (1–5 fractions) of radiation therapy to longer courses (more than 10 fractions) of radiation. Yet, the development of rapid access clinics at the RRRP and the Vancouver Rapid Access (VARA, Vancouver, BC, Canada) clinic have had different results. There was no significant change in practice patterns for palliation of lung cancer with symptomatic disease in the chest at the RRRP (39). Thirteen different fractionation schemas were in use. Sixty percent of patients completed treatment within 1 week, which contrasts to practice patterns in the US, where most patients have longer courses of RT (40).
In contrast, at VARA a clinic designed for patients with metastatic lung cancer decreased the time interval between referral and consult and increased the proportion of patients treated on the same day (or within 3 days) of consultation (19). Same day radiation therapy was given to 72% of patients compared with 41% who received usual care. Hypofractionated RT was used in 73% of patients requiring intrathoracic palliation, 94% of patients treated for bone metastasis and 80% of patients treated for brain metastasis. One reason for this difference may be that the VARA clinic was staffed by 4 physicians with lung cancer expertise and an interest in palliative radiation oncology.
More than half of patients at VARA were referred for additional services (home care nursing, palliative care, counseling) compared with 31% of patients receiving usual care. Fewer patients were double booked into an oncologists’ schedule (13% vs. 23%). Radiation oncologists staffing VARA were surveyed about the benefits. They felt that this clinic enabled a more streamlined process for patients treated with radical and palliative intents, created a more balanced workflow and allowed them to spend more time assessing the needs of patients who needed palliative radiation therapy. For patients with lung cancer treated for bone metastasis, 59% were treated with a SF and an additional 35% with 20 Gy in 5 fractions. For those treated with brain metastasis, 80% were treated with 5 or fewer fractions. For those treated for symptomatic chest disease, 73% received 5 or fewer fractions of radiation therapy.
Patients with brain metastases
The RAPRP brain metastasis clinic incorporates a multidisciplinary approach to the care of patients with brain metastasis (17). In the initial feasibility study of 44 patients, the median time from referral to clinic appointment was 6 days and 76% of patients were seen within 1 week. Ninety-four percent of patients began brain radiation therapy on the day of their initial consultation. Between one third and 45% of patients required additional assessments by the multi-disciplinary team (social work, dietician, occupational therapy).
By integrating palliative care expertise into the routine care of patients with brain metastasis in the VARA, increases were seen in the number of patients with advanced care planning at the first visit, and less use of radiation therapy at the end of life (12). Radiation therapy use within 14 days of death decreased from 9% to 1% and within 30 days of death declined from 19% to 6%. Twenty-five patients did not undergo radiation therapy and 40% of these patients died within 30 days of consultation. The choice not to undergo brain radiation therapy was likely influenced by more frank discussion of prognosis, goals of care and likelihood of benefit from whole brain radiation due to the integration of palliative care consultation. Together with the low rates of end of life RT, this likely demonstrates appropriate selection of patients for radiation therapy to maximize the clinical benefit of brain radiation and avoid the significant associated morbidity in patients with limited life expectancy.
Other outcomes
Advanced directives
Given the population of people served in a palliative radiation oncology service, addressing end of life decisions is an important component. It is well documented that patients who have advanced directives and palliative care input receive less aggressive and inappropriate care at the end of life (36). It is also important, that any advanced directives and decisions about resuscitation be clearly documented in a patient’s medical record to avoid unwanted aggressive care for a medical emergency. A review of patients newly referred to the RRRP revealed that only 6% of patients had clear documentation of code status (41). The proportion of patients with a DNR code status documented increased from 3.8% to 16.5% from 2004 to 2013 in the RRRP. In 2013, only 4 patients did not have documentation of their code status at the time of their first visit. All four had code status documented at a subsequent visit (29). At the University of Pennsylvania, a “palliative radiation oncology psychosocial care plan” (PRO PCP) assesses functional status, psychological coping, addresses advanced directives and encourages patients to reflect on their goals of care as well as their hopes, fears and wishes (32). Implementation of the Palliative Radiation Oncology at Vanderbilt (PROV) service resulted in an increase in documentation of goals of care from 5% to 60% (14). Similarly there was an increase in the involvement of the palliative care team from 15% to 60%.
Communication with referring physicians and patients
Referring physicians
Communication back to the referring physician is an essential part of the consultative process. This is especially true in more rural areas where a physician less comfortable with the side effects of radiation may be managing the majority of a patient’s care. A standardized form was developed at the RRRP and has been used elsewhere to communicate with the referring physicians on the day of consultation (42). This brief form contains a description of the radiation treatment recommendations along with the expected side effects and goal of treatment. It is filled out at the time of the consult and faxed to the referring physician on the same day; the full consultation note is delivered within 2 weeks.
Easing the burden of follow-up with telephone communication
Many patients with advanced cancer have limited life expectancy and high symptom burdens and travel to the radiation therapy clinic can be very uncomfortable. Compliance with physical clinic visits is therefore an issue for follow-up after palliative radiation therapy. Chow et al. at the RRRP developed a telephone follow-up protocol, which has since been implemented elsewhere (43). During their initial feasibility study, 41% of patients died within 12 weeks. Surviving patients were called at 1, 2, 4, 8 and 12 weeks after completion of radiation therapy. Between 63% and 68% of patients answered the telephone surveys. When their 3-year results were reviewed, 31% died and response rates for surviving patients ranged from 48–57% (44).
Enhanced medication management and counseling
Given traditional clinic time limitations, detailed attention to medication management is often deferred. Yet, many patients are unclear about how to take prescribed medications, are overwhelmed with the complexity of short- and long-term opioid dosing or experience common side effects which often can be mitigated with proper counseling, e.g., constipation with opioid use. Involvement of a clinical pharmacist enabled screening for opioid toxicity (88%), medication counseling (84%), and medication changes (29%). Medication counseling services included discussions about bowel regimens (86%) and hydration status (41%). The clinical pharmacist identified opportunities for therapeutic intervention in 43% of patients. These interventions included compliance issues, proper dosing and use, side effect identification and management (16,45).
Referrals for other needed care
Given the high symptom burden of patients with advanced cancer and a patient’s capacity to process information, there is a limit to what can be addressed in a single visit by a single clinician. Patients with advanced cancer often need multiple additional services. Rapid access clinics allow enhanced opportunities to quantify the additional needs of their patients and refer them for appropriate services. The proportion of patients who were referred for other services, e.g., home nursing care, increased from 31% to 54% (P=0.01) after implementation of the VARA for lung cancer palliation (19). In the RAPRP brain metastasis clinic at Cross Cancer Institute, between 33–45% of patients were referred for social work evaluation, occupational therapy and/or a nutritional evaluation (17,20). In patients seen by multiple clinicians, multidisciplinary recommendations were made for most patients seen by the pharmacist, the occupational therapist, the registered dietician and/or the social worker (20).
Academic outcomes
Rates of clinical trial accrual vary by center and are particularly problematic in palliative cancer care where many patients have limited life expectancy and high symptom burden. Yet, there are many relevant research questions to be answered in this patient population. By performing prospective trials of patients with advanced cancer, symptom burdens may be reduced, and care processes and delivery can be enhanced. By simplifying clinical trial design and employing a dedicated research assistant the RRRP increased the proportion of patients enrolled in clinical trials from 14% to 60% (46,47). At the RAPRP brain metastasis clinic, 91% of patients participated in a research study (17).
In 2004 the RRRP began a program to introduce undergraduate students to the clinical environment and provide opportunities to conduct clinical research (48). Over the decade from 2004–2013, 54 college students participated in the RRRP which resulted in 215 first authorship articles, 43% of which are available on PubMed. In addition, students wrote 40 book chapters, received 58 invited presentations and 99 awards and delivered 136 oral presentations.
Multidisciplinary palliative radiation oncology palliative grand rounds became part of the RRRP in 1998 as a continuing medical education initiative. More than 87–91% of those surveyed found the material relevant, interesting and instructional (49).
Conclusions
Palliative radiation oncology programs developed in response to issues of access and fragmentation. Key considerations for the design of a new palliative radiation program are outlined in Table 2. Programs needs to be tailored to the patient population they intend to serve while factoring in the unique talents and challenges of their institution. Program metrics are key to the design process as they allow comparison with existing programs and continual improvement. There is still work to be done to design radiotherapy courses as short as needed to achieve the desired goals (33). SF utilization has improved for the treatment of bone metastasis with the development of rapid access clinics but use of multi-fraction regimens persists in most settings. Similarly, the use of hypofractionated radiation therapy for other indications have increased with the implementation of rapid access and enhanced palliative radiation therapy programs.
Palliative radiation therapy programs have improved patient access and timeliness of the radiation consult to treatment process. Additional opportunities for improvement include continued attention to addressing and documenting advanced directives and accruing patients to clinical trials. These programs enhance multidisciplinary care and connect patients with other important services. They have responded to the needs of their patients and medical systems and have succeeded in improving access and care for patients with advanced cancer.
Acknowledgments
Thanks to Dr. Shayna Rich for her feedback on an early draft of this article.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Samant RS, Fitzgibbon E, Meng J, et al. Barriers to palliative radiotherapy referral: a Canadian perspective. Acta Oncol 2007;46:659-63. [Crossref] [PubMed]
- Bansal M, Patel FD, Mohanti BK, et al. Setting up a palliative care clinic within a radiotherapy department: a model for developing countries. Support Care Cancer 2003;11:343-7. [PubMed]
- Andersson L, Chow E, Finkelstein J, et al. The ultimate one-stop for cancer patients with bone metastases: new combined bone metastases clinic. Can Oncol Nurs J 1999;9:103-4. [PubMed]
- Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer 2006;14:38-43. [Crossref] [PubMed]
- Danjoux C, Szumacher E, Andersson L, et al. Palliative radiotherapy at Toronto-Sunnybrook regional cancer centre: the rapid response radiotherapy program. Curr Oncol 2000;7:52-6.
- de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer 2009;17:757-62. [Crossref] [PubMed]
- Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program: a review of 2008-2012. Curr Oncol 2013;20:e206-11. [Crossref] [PubMed]
- Holt TR, Yau VK. Innovative program for palliative radiotherapy in Australia. J Med Imaging Radiat Oncol 2010;54:76-81. [Crossref] [PubMed]
- Gorman D, Balboni T, Taylor A, et al. The Supportive and Palliative Radiation Oncology Service: A Dedicated Model for Palliative Radiation Oncology Care. J Adv Pract Oncol 2015;6:135-40. [PubMed]
- Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol 2014;4:247-53. [Crossref] [PubMed]
- Wu JS, Kerba M, Wong RK, et al. Patterns of practice in palliative radiotherapy for painful bone metastases: impact of a regional rapid access clinic on access to care. Int J Radiat Oncol Biol Phys 2010;78:533-8. [Crossref] [PubMed]
- Jung H, Sinnarajah A, Enns B, et al. Managing brain metastases patients with and without radiotherapy: initial lessonsfrom a team-based consult service through a multidisciplinary integrated palliative oncology clinic. Support Care Cancer 2013;21:3379-86. [Crossref] [PubMed]
- Chang S, May P, Goldstein NE, et al. A Palliative Radiation Oncology Consult Service Reduces Total Costs During Hospitalization. J Pain Symptom Manage 2018;55:1452-8. [Crossref] [PubMed]
- Stavas MJ, Pagan JD, Varma S, et al. Building a palliative radiation oncology program: From bedside to B.E.D. Pract Radiat Oncol 2017;7:203-8. [Crossref] [PubMed]
- Schuster JM, Smith TJ, Coyne PJ, et al. Clinic offering affordable radiation therapy to increase access to care for patients enrolled in hospice. J Oncol Pract 2014;10:e390-5. [Crossref] [PubMed]
- Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer 2009;17:163-70. [Crossref] [PubMed]
- Danielson B, Fairchild A. Beyond palliative radiotherapy: a pilot multidisciplinary brain metastases clinic. Support Care Cancer 2012;20:773-81. [Crossref] [PubMed]
- Casson C, Johnson J. Implementation and evaluation of a rapid access palliative clinic in a New Zealand cancer centre. J Med Radiat Sci 2014;61:217-24. [Crossref] [PubMed]
- Lefresne S, Berthelet E, Cashman R, et al. The Vancouver rapid access clinic for palliative lung radiation, providing more than just rapid access. Support Care Cancer 2015;23:125-32. [Crossref] [PubMed]
- Pituskin E, Fairchild A, Dutka J, et al. Multidisciplinary team contributions within a dedicated outpatient palliative radiotherapy clinic: a prospective descriptive study. Int J Radiat Oncol Biol Phys 2010;78:527-32. [Crossref] [PubMed]
- Fitzgibbon EJ, Samant R, Meng J, et al. Awareness and use of the Rapid Palliative Radiotherapy Program by family physicians in Eastern Ontario: a survey. Curr Oncol 2006;13:27-32. [PubMed]
- Hartsell WF, Konski AA, Lo SS, et al. Single fraction radiotherapy for bone metastases: clinically effective, time efficient, cost conscious and still underutilized in the United States? Clin Oncol (R Coll Radiol) 2009;21:652-4. [Crossref] [PubMed]
- Ellsworth SG, Alcorn SR, Hales RK, et al. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys 2014;89:1100-5. [Crossref] [PubMed]
- Thavarajah N, Zhang L, Wong K, et al. Patterns of practice in the prescription of palliative radiotherapy for the treatment of bone metastases at the Rapid Response Radiotherapy Program between 2005 and 2012. Curr Oncol 2013;20:e396-405. [Crossref] [PubMed]
- ABIM. Choosing Wisely. 2012.
- Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:4-12. [Crossref] [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- Skamene S, Agarwal I, Makar M, et al. Impact of a dedicated palliative radiation oncology service on the use of single fraction and hypofractionated radiation therapy among patients with bone metastases. Ann Palliat Med 2018;7:186-91. [Crossref] [PubMed]
- Pulenzas N, Lechner B, Zhang L, et al. The incidence of DNR documentation in patients referred for palliative radiotherapy in the Rapid Response Radiotherapy Program. J Palliat Med 2014;17:1296-7. [Crossref] [PubMed]
- Hoegler D, Thomas G, Stevenson C. Radiation Oncology. Clin Invest Med 1997;20:84.
- Wu JS, Wong RK, Lloyd NS, et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases - an evidence-based practice guideline. BMC Cancer 2004;4:71. [Crossref] [PubMed]
- Cammy R. Developing a Palliative Radiation Oncology Service Line: The Integration of Advance Care Planning in Subspecialty Oncologic Care. J Soc Work End Life Palliat Care 2017;13:251-65. [Crossref] [PubMed]
- Mackillop WJ. The principles of palliative radiotherapy: a radiation oncologist's perspective. Can J Oncol 1996;6:5-11.
- Balboni T, Balboni M, Paulk ME, et al. Support of cancer patients' spiritual needs and associations with medical care costs at the end of life. Cancer 2011;117:5383-91. [Crossref] [PubMed]
- May P, Garrido MM, Cassel JB, et al. Cost analysis of a prospective multi-site cohort study of palliative care consultation teams for adults with advanced cancer: Where do cost-savings come from? Palliat Med 2017;31:378-86. [Crossref] [PubMed]
- Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 2008;26:3860-6. [Crossref] [PubMed]
- Ford A, Bydder S, Ebert MA. The use of On-Board Imaging to plan and deliver palliative radiotherapy in a single cohesive patient appointment. J Med Imaging Radiat Oncol 2011;55:633-8. [Crossref] [PubMed]
- Wong RK, Letourneau D, Varma A, et al. A one-step cone-beam CT-enabled planning-to-treatment model for palliative radiotherapy-from development to implementation. Int J Radiat Oncol Biol Phys 2012;84:834-40. [Crossref] [PubMed]
- Fairchild A, Goh P, Sinclair E, et al. Has the pattern of practice in the prescription of radiotherapy for the palliation of thoracic symptoms changed between 1999 and 2006 at the rapid response radiotherapy program? Int J Radiat Oncol Biol Phys 2008;70:693-700. [Crossref] [PubMed]
- Coia LR, Hanks GE, Martz K, et al. Practice patterns of palliative care for the United States 1984-1985. Int J Radiat Oncol Biol Phys 1988;14:1261-9. [Crossref] [PubMed]
- Bradley NM, Sinclair E, Danjoux C, et al. The do-not-resuscitate order: incidence of documentation in the medical records of cancer patients referred for palliative radiotherapy. Curr Oncol 2006;13:47-54. [PubMed]
- Barnes EA, Chow E, Andersson L, et al. Communication with referring physicians in a palliative radiotherapy clinic. Support Care Cancer 2004;12:669-73. [PubMed]
- Chow E, Wong R, Connolly R, et al. Prospective assessment of symptom palliation for patients attending a rapid response radiotherapy program. feasibility of telephone follow-up. J Pain Symptom Manage 2001;22:649-56. [Crossref] [PubMed]
- Chow E, Fung KW, Bradley N, et al. Review of telephone follow-up experience at the Rapid Response Radiotherapy Program. Support Care Cancer 2005;13:549-53. [Crossref] [PubMed]
- Gagnon L, Fairchild A, Pituskin E, et al. Optimizing pain relief in a specialized outpatient palliative radiotherapy clinic: contributions of a clinical pharmacist. J Oncol Pharm Pract 2012;18:76-83. [Crossref] [PubMed]
- Bradley NM, Chow E, Tsao MN, et al. Reasons for poor accrual in palliative radiation therapy research studies. Support Cancer Ther 2006;3:110-9. [Crossref] [PubMed]
- Lien K, Zeng L, Bradley N, et al. Poor Accrual in Palliative Research Studies: An Update From the Rapid Response Radiotherapy Program. World J Oncol 2011;2:217-24. [PubMed]
- McDonald R, Lechner B, Pulenzas N, et al. Student Accomplishments in the Rapid Response Radiotherapy Program: A 10-Year Review. J Cancer Educ 2015;30:693-8. [Crossref] [PubMed]
- Szumacher E, Franssen E, Hayter C, et al. Multidisciplinary radiation oncology palliative care rounds as a continuing educational activity implementing the rapid response radiotherapy program at the toronto sunnybrook regional cancer centre. J Cancer Educ 2003;18:86-90. [Crossref] [PubMed]


