
Validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: extra-cranial disease progression is an independent risk factor
Introduction
Central nervous system (CNS) metastases are a serious complication of solid organ tumours, resulting in significant morbidity and mortality (1). Breast cancer (BC) is the second most common cause of brain metastases (BM) (2). Previously regarded as having a uniformly dismal prognosis, advances in treatment have resulted in the recognition that BC patients with BM are heterogeneous with widely differing survival (3-5). Factors found to prognosticate survival in these patients include performance status, age, number of BM, tumour histology, receipt of local or systemic therapy and extra-cranial disease status (6-10). Various prognostic instruments incorporating these factors have been developed (11-14).
The Graded Prognostic Assessment (GPA) is one of the better-known prognostic instruments (15).
Originally developed from a database of 1,960 patients with BM accrued from five Radiation Therapy Oncology Group (RTOG) trials, this instrument was notable for its exclusion of systemic disease control as a variable as the authors viewed the lack of precision in determining “disease control” a limitation (16-19). The GPA was further refined in subsequent years. Starting with the development of disease-specific GPA (DS-GPA) for specific cancer subtypes using a multi-institutional analysis of 4,259 patients with BM, the authors went on to refine the Breast Cancer Specific GPA (B-GPA) by analysing a sample of 400 patients with BC and BM (20-22). Further modification to produce the Modified Breast-GPA (mB-GPA) has recently been proposed by integrating the number of BM as an additional variable (23).
The primary aim of our study is to validate the B-GPA and mB-GPA in Asian BC patients treated at a single tertiary institute.
The secondary aim is to explore extra-cranial disease progression as a prognostic factor in our population as patients with BM often have co-existing extracranial disease that can have major impact on survival, regardless of intracranial disease control (24).
Methods
Female patients 18 years or older with histologically proven BC and newly diagnosed intra-parenchyma referred for radiotherapy between 1st January 2006 and 31st October 2017 were retrospectively identified from an institutional database. Patients diagnosed before 2006 were excluded as HER-2 status was not routinely tested for and Trastuzumab was not widely available. Patients with incomplete information or recurrent BM were excluded.
Clinical and biological information including patient demographics, Karnofsky Performance Score (KPS), treatment received, extra-cranial disease progression and other tumour characteristics were extracted from patient’s medical records.
Patients were classified into four BC subtypes: “Basal” (ER−/PR−/HER2−), “Luminal A” (ER and/or PR+, HER2−), “Luminal B” (ER and/or PR+, HER2+) and “HER2 Enriched” (ER/PR−, HER2+). Overall survival (OS) was measured from date of radiotherapy completion to the date of death. If no treatment was given, OS was measured from date of BM diagnosis to the date of death. Patients without events were censored at the date of their last follow-up.
We counted the number of BM based on best available brain imaging or radiological reports. Fine-cut MRIs were preferred over CT-scans. We further classified patients based on their extra-cranial disease status. We determined extra-cranial disease status from radiology reports and/or physician assessment documented in clinical notes. Three categories are recognised. Patients with progressive systemic disease seen on concurrent staging scans whilst on systemic therapy were termed “extra-cranial disease progression”. Patients with systemic disease status of complete response, partial response or stable disease whilst on systemic therapy were labelled “extra-cranial disease control”. Patients who were treatment naive or who had just started treatment without subsequent follow-up scans were termed “newly diagnosed metastatic disease” (25).
Ethics
This study was approved by Singhealth Central Institutional Review Board (CIRB Ref No. 2013/1037/B). Waiver of consent was granted considering the retrospective, non-interventional nature of study.
Statistical analysis
We calculated the B-GPA and mB-GPA scores for each patient and divided the patients into four bands (0.0–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0), similar to prior work by the original GPA developers (20,23).
Univariate (UVA) and multivariate analysis (MVA) using the Cox proportional hazard model were performed to investigate the factors prognostic of OS. The Kaplan-Meier method was used to estimate OS. Analysis was stratified for patients by GPA score bands and log-rank test was used to compare survival between bands. Harrell’s concordance index (C-index) was used to assess the discriminating ability of the B-GPA and mB-GPA within our population and Akaike information criterion (AIC) used to compare between models.
Statistical analyses were carried out using IBM SPSS Statistics (version 25. IBM Corp., Armonk, NY, USA) and R software (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria) (26). The survival and survminer packages were utilized to generate the C-index and survival plots respectively. All reported P values were two-sided with significance level set at 5% (P<0.05).
Results
We identified 360 BC patients with BM from January 2006 to October 2017, of which 282 patients satisfied our inclusion criteria. Fifty patients were excluded as they had recurrent BM and 28 were excluded due to incomplete data. Patient and tumour characteristics are summarised in Table 1.
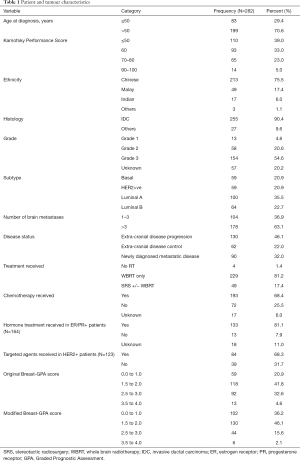
Full table
The median age at BM diagnosis was 54.5 (IQR, 48.0 to 61.0) years, with 199 patients (70.6%) older than 50 years old. Our population was predominantly Chinese (75.5%). Overall, 110 patients (39.0%) had a KPS of ≤50 and only 14 patients (5.0%) with a KPS of 90–100. We found no significant difference in KPS distribution based on tumour subtype (P=0.88). Patients with extra-cranial disease progression had significantly lower KPS compared to those without extra-cranial disease progression (P<0.05). A large proportion of our population had received prior treatment: 68.4% had chemotherapy, 81.1% of hormone-receptor positive patients had hormonal therapy and 68.3% of HER2 positive patients had targeted therapy. In our cohort, 130 (46.1%) had extra-cranial progression despite receiving standard systemic treatment.
Median follow-up was 4.93 months (IQR, 1.74 to 13.01 months). At the time of analysis, 266 (94.3%) patients had died of which 47 (16.7%) died within 1 month of radiotherapy. Out of the 47 patients, 35 (74.5%) had KPS <50, 35 (74.5%) were >50 years of age, 30 (63.8%) had more than 3 brain metastases and 32 (68.1%) had extra-cranial disease progression. Patients who died within 1 month had significantly lower KPS and a higher proportion had extracranial disease progression in comparison to the rest of the cohort (P<0.05).
All components of the mB-GPA (KPS, age, number of BM and tumour subtype) were significantly associated with survival in multivariate analysis (MVA) (Table 2). Additionally, we found extra-cranial progression to be significantly associated with survival in MVA (P<0.001).
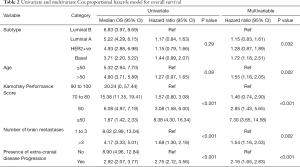
Full table
Patients were grouped based on their B-GPA and mB-GPA scores (Tables 1 and 3). The Kaplan-Meier curve for survival using either scoring systems demonstrated excellent separation between GPA bands (P<0.001) (Figures 1 and 2).
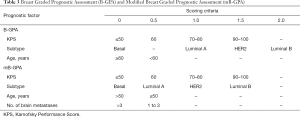
Full table
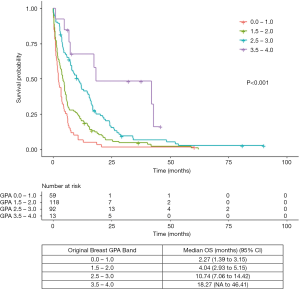
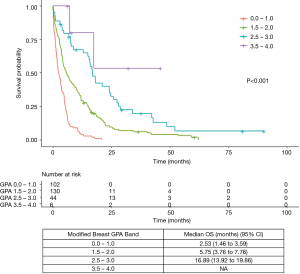
B-GPA
Median OS for patients with B-GPA band 0.0–1.0 was 2.27 months, compared to 4.04, 10.74 and 18.27 months for B-GPA band 1.5–2.0, 2.5–3.0 and 3.5–4.0 respectively (Figure 1). C-index was 0.64 and AIC was 2,483.39 for B-GPA.
mB-GPA
Median OS for patients with mB-GPA band of 0.0–1.0 was 2.53 months, compared to 5.75 and 16.89 months for mB-GPA band of 1.5–2.0 and 2.5–3.0 respectively. Estimates of OS for patients with mB-GPA band of 3.5–4.0 was imprecise as there were only 6 patients with 2 events (Figure 2). The mB-GPA demonstrated marginally better discrimination than the B-GPA with a higher C-index of 0.65 and lower AIC of 2,445.78.
Extra-cranial disease progression
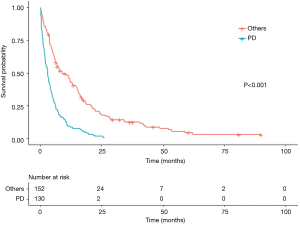
Extra-cranial progression was shown to be a significant predictor of survival on univariate and MVA (multivariate HR 2.16, 95% CI, 1.65–2.83). Patients with extra-cranial progression had a significantly lower median OS of 2.92 months (95% CI, 2.07–3.77) while those with controlled extra-cranial disease or newly diagnosed metastatic disease had a median OS of 8.90 months (95% CI, 4.96–12.84) (Figure 3). Including extra-cranial progression as a factor within the mB-GPA model improved its C-index and AIC to 0.69 and 2,419.58 respectively.
Discussion
BC patients with BM are heterogeneous with markedly different survival (3,4,24). Many treatment modalities are available in the care of these patients. The appropriate choice is guided to a significant extent by prognostication.
For patients with favourable prognosis, treatment may include surgery with or without adjuvant radiation, stereotactic radiosurgery (SRS) with or without whole brain radiotherapy (WBRT) or WBRT alone. Patients with poor prognosis may be appropriate for only a short course of WBRT or just supportive care alone as WBRT is not without toxicities and the extent of its clinical benefit remains poorly defined in patients with short survival (27,28). WBRT is known to cause acute toxicities which resolve only gradually with time and tumour shrinkage resulting in clinical improvement that is not immediate. Hence, only patients who can outlive the time required for toxicities to resolve and for clinical improvement to manifest can benefit from WBRT.
For patients who are not expected to live long yet have symptomatic BM requiring WBRT, a shorter course of 12 Gy in 2 daily fractions may be more appropriate and was proven in a British trial not to be inferior to a longer regimen (29). A prospective trial on patients with non-small cell lung cancer with BM who were unsuitable for resection or radiosurgery also showed little overall additional benefit of WBRT over best supportive care. However, improved survival for WBRT was shown for patients younger than 60 years and there was a trend for better outcome in patients with better performance status (30). A similar trial for BC is as yet unavailable.
In our cohort, 47 (16.7%) of our patients had a median survival of less than 1 month after radiotherapy. These patients tended to have poorer function and extra-cranial disease progression. A survival of less than 1 month after cancer treatment is an indicator of poor quality of life (27). Clinicians need to be able to determine a subpopulation of patients whose prognosis remains so guarded that best supportive care is more appropriate.
The original B-GPA model consisted of three factors: KPS, age and tumour subtype (20). It was externally validated in a large cohort of 1,552 BC patients with newly diagnosed BM from 1996 to 2013. Within this validation study, Subbiah et al. proposed a modified model by integrating number of BM as a variable. Developed using multivariable Cox regression and recursive partitioning analysis as per prior GPA models, the C-index for the original B-GPA was 0.78 (95% CI, 0.77–0.80) while the C-index for the proposed mB-GPA was 0.84 (95% CI, 0.83–0.85) (23). It has since received two independent external validations (31,32).
Our study confirms the prognostic value of the individual components of the B-GPA (KPS, age and tumour subtype). Within tumour subtype, only the “Basal” subtype was associated with a significantly increased HR with reference to “Luminal B” (HR 1.72, 95% CI, 1.18–2.51). This ran contrary to findings from prior large population studies (9,33,34). We hypothesize that this may be because a large proportion of our population had already been heavily pre-treated and were refractory to further systemic therapy by the time of referral for radiotherapy (68.3% had targeted therapy, 81.1% had hormonal treatment, 68.4% had chemotherapy). A total of 46.1% of patients also had documented extra-cranial disease progression on treatment. Thus, the “survival benefit” conferred by subtype could have been muted. We found the number of BM to be a significant prognostic factor, which affirms its inclusion into the mB-GPA (31,32).
We have demonstrated that both B-GPA and mB-GPA are moderately successful in discriminating between OS of Asian BC patients with BM. The mB-GPA performed marginally better than the B-GPA with a lower AIC and higher C-index. Our findings echo that of an earlier European multi-centre external validation study in which the authors found a C-index of 0.64 and 0.66 for B-GPA and mB-GPA respectively (31). This is considerably lower than the adjusted C-index of 0.80 proposed during internal validation (23). A C-index of ≤0.70 lacks clinically acceptable discrimination and we suggest that additional model refinement may improve its prognostic value in BC patients with BM (35).
Extra-cranial progression has been shown in multiple studies to have a significant impact on survival and was included in several prognostic indices (6,16-18). However, this factor was excluded from analysis during initial scale creation. We agree with Sperduto et al. initial argument that “estimation of systemic disease is fraught with inconsistency due to the variation in type and timing of imaging studies” (15,19). However, we do not agree that factors which produce variability should be removed prematurely before careful consideration of its clinical importance. Firstly, clinical variables such as performance status, delirium and dyspnea are examples of factors that has a certain degree of subjectivity and interrater variability (36,37). Nonetheless, they remain of significant prognostic importance and their “inconsistency in assessment” does not preclude successful incorporation within useful prognostic indices (38-40). Secondly, recent efforts in the development of the Response Evaluation Criteria in Solid Tumors (RECIST) have provided reliable and validated methods to standardise assessment of response in solid tumours (41,42). Thirdly, clinical reasoning suggests that in a patient receiving specific treatments, the disease response is a critical aspect of prognostication. Patients who demonstrate extra-cranial progression despite best efforts have limited options for further systemic therapy. These patients tend to die from uncontrolled systemic disease independent of intracranial control (24,43-45).
In our population, patients with extra-cranial progression had significantly poorer prognosis compared to the rest of the cohort (2.92 vs. 8.90 months) and maintained its independent prognostic value at MVA. Incorporation of extra-cranial progression as a variable improved C-index and AIC although we are aware that presence of over-fitting needs to be first verified on subsequent validation studies.
Our study has several strengths. To the best of our knowledge, this is the first validation of mB-GPA within the Asian population. We had a relatively large cohort of patients with high event rates and few losses to follow-up. There was accurate documentation of treatment received and systemic disease control.
However, our study had several limitations. Firstly, due to the retrospective nature of this study, it suffers from inherent flaws such as selection bias, missing data and reliance on the accuracy of clinical records and data captured by our institutional database. Secondly, treatment patterns captured in this historic cohort may not reflect current practice trends. A proportion of our patients declined standard of care treatment which may differ depending on centres. Thus, this limits generalizability of our results. Thirdly, our institutional database comprised of only patients with symptomatic BM referred to the Radiation Oncology Department for consideration of radiotherapy. Thus, we were unable to include patients with less symptomatic and smaller BM who underwent systemic therapy alone. Lastly, a small proportion of our patients achieved the highest GPA band of 3.5–4.0 as the patients in our study tended to be older and had poorer performance status. We were unable to adequately analyse this subgroup of patients.
Conclusions
Our results show that mB-GPA is marginally more discriminating than B-GPA and both scores display moderate abilities in stratifying survival in BC patients with BM. In addition, we strongly propose the inclusion of extra-cranial disease progression as a factor in future model development due to its significant impact on survival.
Acknowledgments
We would like to thank the multi-disciplinary team involved in the care of these patients and for their contribution towards the Singapore Health Services Joint Breast Cancer Registry. We would like to thank Dr. Sim Yirong and Adj Assoc Prof Benita Tan for their clinical input and advice. We would also like to thank Mr. John Lim Heng Chi for his invaluable statistical input.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Singhealth Central Institutional Review Board (CIRB Ref No. 2013/1037/B). Waiver of consent was granted considering the retrospective, non-interventional nature of study.
References
- Giglio P, Gilbert MR. Neurologic Complications of Cancer and its Treatment. Curr Oncol Rep 2010;12:50-9. [Crossref] [PubMed]
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48-54. [Crossref] [PubMed]
- Martin AM, Cagney DN, Catalano PJ, et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol 2017;3:1069-77. [Crossref] [PubMed]
- Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 2010;21:942-8. [Crossref] [PubMed]
- Lin NU, Bellon JR, Winer EP. CNS Metastases in Breast Cancer. J Clin Oncol 2004;22:3608-17. [Crossref] [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [Crossref] [PubMed]
- Braccini AL, Azria D, Thezenas S, et al. Prognostic factors of brain metastases from breast cancer: impact of targeted therapies. Breast 2013;22:993-8. [Crossref] [PubMed]
- Lentzsch S, Reichardt P, Weber F, et al. Brain metastases in breast cancer: prognostic factors and management. Eur J Cancer 1999;35:580-5. [Crossref] [PubMed]
- Leone JP, Leone J, Zwenger AO, et al. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer 2017;74:17-25. [Crossref] [PubMed]
- Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 2008;112:2359-67. [Crossref] [PubMed]
- Braccini A-L, Azria D, Thezenas S, et al. Comparative performances of prognostic indexes for breast cancer patients presenting with brain metastases. BMC Cancer 2013;13:70. [Crossref] [PubMed]
- Laakmann E, Riecke K, Goy Y, et al. Comparison of nine prognostic scores in patients with brain metastases of breast cancer receiving radiotherapy of the brain. J Cancer Res Clin Oncol 2016;142:325-32. [Crossref] [PubMed]
- Nieder C, Marienhagen K, Astner ST, et al. Prognostic scores in brain metastases from breast cancer. BMC Cancer 2009;9:105. [Crossref] [PubMed]
- Tabouret E, Metellus P, Gonçalves A, et al. Assessment of prognostic scores in brain metastases from breast cancer. Neuro-Oncol 2014;16:421-8. [Crossref] [PubMed]
- Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: the Graded Prognostic Assessment. J Neurosurg 2008;109 Suppl:87-9. [Crossref] [PubMed]
- Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 2000;46:1155-61. [Crossref] [PubMed]
- Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys 2004;60:218-24. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 2012;82:2111-7. [Crossref] [PubMed]
- Subbiah IM, Lei X, Weinberg JS, et al. Validation and Development of a Modified Breast Graded Prognostic Assessment As a Tool for Survival in Patients With Breast Cancer and Brain Metastases. J Clin Oncol 2015;33:2239-45. [Crossref] [PubMed]
- Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol 2013;14:e396-406. [Crossref] [PubMed]
- Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 2009;100:894-900. [Crossref] [PubMed]
- R Core Team. R: A language and environment for statistical computing, 2013.
- Jones JA, Lutz ST, Chow E, et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin 2014;64:296-310. [Crossref] [PubMed]
- Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:2804-7. [Crossref] [PubMed]
- Priestman TJ, Dunn J, Brada M, et al. Final results of the Royal College of Radiologists’ trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996;8:308-15. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Griguolo G, Jacot W, Kantelhardt E, et al. External validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: A multicentric European experience. Breast 2018;37:36-41. [Crossref] [PubMed]
- Tai CH, Wu CC, Hwang ME, et al. Single institution validation of a modified graded prognostic assessment of patients with breast cancer brain metastases. CNS Oncol 2018;7:25-34. [Crossref] [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [Crossref] [PubMed]
- Haque R, Ahmed SA, Inzhakova G, et al. Impact of Breast Cancer Subtypes and Treatment on Survival: An Analysis Spanning Two Decades. Cancer Epidemiol Biomarkers Prev 2012;21:1848-55. [Crossref] [PubMed]
- David WH, Lemeshow S. Assessing the Fit of the Model. In: Applied Logistic Regression. Wiley-Blackwell, 2005:143-202.
- Shi Q, Warren L, Saposnik G, et al. Confusion assessment method: a systematic review and meta-analysis of diagnostic accuracy. Neuropsychiatr Dis Treat 2013;9:1359-70. [Crossref] [PubMed]
- Chow R, Chiu N, Bruera E, et al. Inter-rater reliability in performance status assessment among health care professionals: a systematic review. Ann Palliat Med 2016;5:83-92. [Crossref] [PubMed]
- Morita T, Tsunoda J, Inoue S, et al. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 1999;7:128-33. [Crossref] [PubMed]
- Scarpi E, Maltoni M, Miceli R, et al. Survival Prediction for Terminally Ill Cancer Patients: Revision of the Palliative Prognostic Score with Incorporation of Delirium. Oncologist 2011;16:1793-9. [Crossref] [PubMed]
- Gwilliam B, Keeley V, Todd C, et al. Development of Prognosis in Palliative care Study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ Support Palliat Care 2015;5:390-8. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ahluwalia MS, Vogelbaum MV, Chao ST, et al. Brain metastasis and treatment. F1000Prime Rep 2014;6:114. [Crossref] [PubMed]
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. [Crossref] [PubMed]
- Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol 2006;24:106-14. [Crossref] [PubMed]


