Cryosurgery for colorectal liver metastases
Introduction
Colorectal cancer ranked second as a cause of death in the western world. The cumulative lifetime risk is approximately 5%, the incident rate in the western world is 50/100,000 (1). Liver metastasis is the main cause of death in the patients with colorectal cancer. Already at the time of detection of the primary tumor, 15-25% of the patients present with liver metastases, another 20% will develop these metastases following treatment of the colorectal primary (2,3). Without any treatment, the median survival after the detection of liver metastases is approximately 9 months, depending on the extent of the disease at the time of diagnosis (4).
In contrast to many other solid tumors, resection of liver metastases from colorectal origin has been shown to result in long-term survival and even cure. In selected patients with metastatic focus confined to the liver, 5-year survival rates are generally reported between 35% and 40%, depending on the extent of liver involvement (5).
However, only few patients are eligible for resection treatment, due to distribution, number and location of lesions, previous liver resections, extrahepatic tumor growth, and general medical status of the patients. Several local ablation techniques, such as radiofrequency, microwave and cryosurgery, have been used to treat unresectable colorectal liver metastases with promising results. Cryoablation is among the first of the thermal ablative techniques that has been widely used as treatment of liver tumors. Unresectable metastases from colorectal cancer are the most frequent indications for cryoablation (6).
Indication
To date, cryosurgical ablation is mainly reserved for treatment of unresectable lesions in patients with colorectal liver metastases (7,8), for example:
- Multiple tumors in both lobes of liver;
- Tumors which locate at anatomical position that does not allow liver resection;
- In patients with co-morbidity and reduced liver reserve for whom resection would have involved too high a risk;
- In patients with resectable lesions who refuse to undergo liver resection, percutaneous cryosurgery is preferably performed.
Technology
As cryosurgery for primary liver cancer, the techniques of cryosurgery include (I) open cryosurgery, which may use cryohepatectomy (cryoablation followed by resection of the cryotreated tumor), or resection of the main tumor plus cryoablation for residual tumor, or cryoablation alone without removing the cryotreated tissue; (II) laparoscopic cryosurgery; and (III) percutaneous cryoablation under guidance of ultrasound or CT.
Clinical results
Colorectal liver metastasis is the most common metastatic disease to the liver. Compared with primary hepatocellular carcinoma, there is larger number of trials of cryosurgery for colorectal liver metastases.
Open cryosurgery
Up to the present, most of cryosurgery for colorectal liver metastases is preformed through intraoperative (laparotomy) approach (Table 1). The median survival following cryosurgery ranges 21-45 months, the 1-, 3-, and 5-year survivals range 52-86%, 10-70%, and 5-44%, respectively.
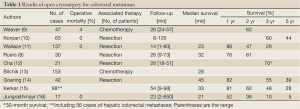
Full Table
Early in 1991, it was Onik et al. (17) who first reported that 18 patients with unresectable colorectal liver metastases underwent cryosurgery, and 22% got complete remission on CT scans and CEA levels during a mean follow-up of 28.8 months. In the same year, Ravikumar et al. (18) summarized their 5-year experience with open cryosurgery for 32 patients with liver tumors, in whom 24 had colorectal liver metastases. After a follow-up period of 5 to 60 months, 28% of patients remained disease free, 34% were alive with disease, and 38% died. Recurrence at the cryotreated site occurred in 9% of patients.
In the following years, several reports had shown survival benefit of patients with colorectal liver metastases following cryosurgery. Sheen et al. (6) reported 41 patients. Median survival was 22 months. Postoperative carcinoembryonic antigen (CEA) levels were significantly reduced. No severe complications were discovered. Kerkar et al. (15) determined the long-term outcomes of cryosurgery in patients with 98 patients with hepatic malignancies including 56 colorectal metastases. Overall survival rates at 1-, 2-, 3-, and 5-year were 81%, 62%, 48%, and 28%, respectively. Median survival was 33 months and median hepatic recurrence-free survival was 20 months. The study pointed out that outcomes depending on tumor type were not significantly different. Weaver et al. (9) determined the effectiveness of cryosurgery as an adjunct to resection in treating 47 patients with colorectal hepatic metastases. The actual survival at 24 months was 62%. Eleven percent of these patients had no evidence of disease at a median follow-up of 30 months. Two years later, Weaver et al. (19) again reported their experience on 158 cryosurgical procedures on 136 patients with unresectable colorectal hepatic metastases. Median survival of all patients was 30 months. Patients with a CEA level >100 ng/dL had a statistically worser survival rate than those with a level <100 ng/dL.
The outcome of patients after cryosurgery often is comparable or superior to conventional resection. Korpan (10) had an interesting prospective and randomized study. One hundred and twenty-three patients with liver metastases in whom more than 60% had colorectal liver metastases were divided into two groups: cryosurgery group and conventional surgical group. The 3-year survival rate was 60% vs. 51% and the 5-year survival rate was 44% vs. 36% in both groups, respectively. There were 19% vs. 8%, respectively, who survived 10 years.
Cryotherapy of the involved or inadequate resection margins after resection of colorectal liver metastases considerably improves local disease control and may allow a greater proportion of patients to undergo potentially curative treatment. Seifert and Morris et al. (20) reported 44 patients with colorectal liver metastases who underwent the freezing for an involved or inadequate resection margin after liver resection. Nineteen patients developed recurrence in the liver but only in 5 cases the recurrence occurred at the resection edge. Median overall and liver disease-free survival was 33 and 23 months, respectively.
Cryosurgery alone or in combination with resection has been proved to improve the survival. Adam et al. (21) reported the outcome of 34 patients with nonresectable liver cancer in whom 25 patients had metastases from colorectal cancer. Cryosurgery was used either as a single treatment or in association with liver resection. There was no intraoperative mortality. Mortality within 2 months was 3%, but was unrelated to the cryosurgery. 2-year cumulative survival was 52%. Brooks et al. (22) commented on the value of synchronous liver resection and cryoablation for colorectal liver metastases in 93 patients for whom complete resection is not possible. Eighty four patients were followed-up for a median of 18 months (range, 1-83 months). 1-, 3- and 5-year survival was 85%, 43% and 19% respectively, with a median survival of 33 months. Onik et al. (23) reported 57 patients with unresectable hepatic metastases who were treated with cryosurgery. Among these cases, 25 (42%) underwent a combination of resection and cryosurgery. The number of lesions treated ranged from 1-16 with a mean of 4.6. Seventy-three per cent of the patients had bilobar disease. Results showed that the disease-free survival rate was 27% during a mean follow-up of 21 months.
Since 2002, Seifert et al. (24) had performed a series of works. In a prospective case-control study 71 patients with liver cancer, in whom 49 cases had colorectal origin and underwent a total of 77 cryosurgical procedures. In 36 of 71 patients, cryosurgery was used in combination with liver resection. Morbidity and hospital mortality were 23% and 2.8%. Seventy-four per cent of patients with colorectal metastases preoperatively elevated CEA returned to normal postoperatively. Tumor recurrence at the cryosite was in 17% of patients. Median survival for all patients and patients with colorectal metastases was 28 and 29 months with a 5-year survival rate of 30% and 33%, respectively. In 2004, Seifert and Junginger (25) summarized the results of the cryosurgery of 77 patients with malignant liver tumors, in whom 55 patients had colorectal liver metastases. Forty patients had cryosurgery only and 37 had an additional liver resection. Median survival was 29 months with a 3- and 5-year-survival rate of 44% and 26%, respectively, for the 55 patients with colorectal liver metastases. In 2005, Seifert et al. (26) compared morbidity and mortality, as well as the recurrence pattern and survival after cryosurgery alone or in combination with resection for 168 patients with colorectal liver metastases. Five-year survival rates following resection and cryotherapy were comparable (23% and 26% respectively), while overall and hepatic recurrence-free survival was inferior following cryotherapy alone.
Percutaneous cryosurgery
There are fewer reports which show percutaneous cryosurgery has results comparable to open cryosurgery for colorectal liver cancer. Huang et al. (27) reported 15 patients who underwent percutaneous cryosurgery for colorectal liver metastases. There was no procedure-related mortality. Liver metastasis growth was significantly delayed for 2 months. Serum CEA levels had an immediate rise, followed by a fall. Mala et al. (28) made a prospective study of 19 patients with colorectal hepatic metastases who underwent 24 cryoablation procedures. There were 16 procedures which were performed percutaneously. Out of 25 ablations, 18 (72%) were assumed adequate. Total ice-ball volume during percutaneous procedures was median 62 cm3 (range, 32-114 cm3). Excellent imaging of the extent of freezing was achieved using MRI. Hospital stay for patients treated percutaneously was median 4 days (range, 3-30 days). No perioperative mortality occurred. Actuarial 2-year tumor-free survival at site of ablation was 48%. At the time of analyses, 92% of patients assumed to be adequately ablated were alive.
We would like to introduce results of our study in details (29). A total of 326 patients with colorectal liver metastases underwent a total of 526 procedures between March 2001 and February 2007. There were 151 patients who underwent repeated procedures of cryosurgery for recurrent tumors in the liver and extrahepatic sites. The results were as follows:
CEA levels
Increased CEA level was observed in 254 patients (77.9%) at the time of the initial diagnosis. Among these patients, CEA level decreased to within the normal range in 197 (77.5%) patients, and increased in 41 patients (16.1%), with no significant change in 16 cases (6.3%), at 3 months after cryosurgery.
Evolution of tumor size
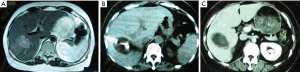
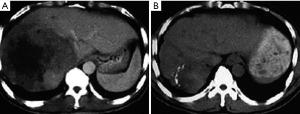
After cryosurgery, an early increase in the size of lesions in relation to the freezing margin >1 cm beyond the limit of the tumor was a constant feature. Cryotreated lesions appeared as hypoechogenic or hypodense areas. Among 280 patients who received CT follow-up, complete response (CR) was observed in 41 patients (14.6%), partial response (PR) in 115 (41.1%), stable disease (SD) in 68 (24.3%), and progressive disease (PD) in 56 patients (20%). Two patients with CR proven by histology and CT are presented in Figures 1,2.
Tumor recurrence
The recurrence rate was 47.2% during a median follow-up of 32 months (range, 7-61 months). Recurrence patterns were presented in Table 2. Sixty-one percent of recurrence was in the liver only and 13.9% in the liver and extrahepatic sites. Extrahepatic recurrence was mainly seen in the lungs and lymph nodes. The recurrence at cryotreated site, including at cryotreated site only as well as cryotreated site and the remaining area of liver, accounted 15.3% of cases who had recurrence and 6.4% of all cases.
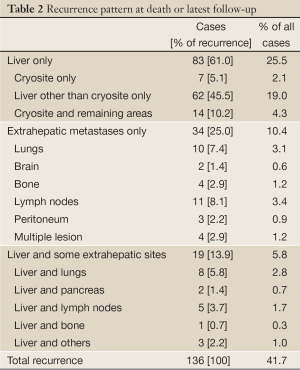
Full Table
Survival
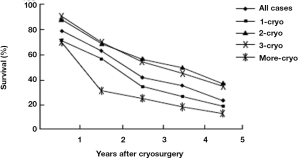
During a median follow-up of 36 months (7-62 months), the median survival of all patients was 29 months (range, 3-62 months). One hundred and ninety six patients (60.1%) died during follow-up, and 130 patients (39.9%) are still alive, with a median survival of 26 and 36 mos, respectively. Overall survival was 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively (Figure 3). Patients with tumor ≤3 cm, tumor in right liver lobe, CEA <100 ng/dL, and postcryosurgery transarterial chemoembolization (TACE) had a higher survival rate. There was no significant difference in terms of survival based on tumor number, pre-cryosurgery chemotherapy, and timing of the development of metastases (synchronous vs. metachronous). Survival was related to the number of cryosurgery procedures performed on the patients. Patients who underwent two or three procedures had an increased survival, compared to those who received cryosurgery once only. However, patients who received cryosurgery on more than three occasions had lower survival (Table 3, Figure 3).
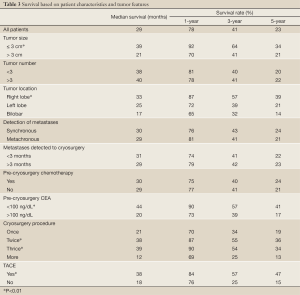
Full Table
Adverse effects
In our 326 cases of liver metastases from colorectal cancer that underwent percutaneous cryosurgery, the minor and major adverse effects of cryosurgery were seen in Table 4. Major adverse effects included hemorrhage from a cryotreated lesion was seen in 5 patients, in whom 3 died of the complication. One patient, who underwent cryosurgery for more than 50% of liver volume, died of hepatic failure. One patient, who received cryosurgery for 8 large metastases, died of a cryoshock syndrome. Three patients suffered from biliary fistula which resolved with transhepatic drainage. Five patients had transient renal insufficiency, which presented increased blood urea nitrogen and creatinine levels for 3-7 days. Two patients developed bacterial hepatic abscess within cryosites and recovered with antibacterial agents and drainage. There were a total of 7 patients who died of the main adverse effects after cryosurgery.
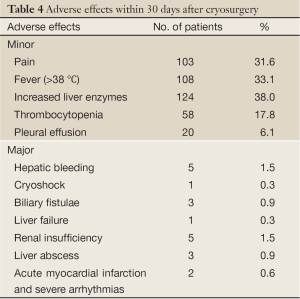
Full Table
Discussion
Rational of combination of cryoablation with resection
It is difficult to draw definitive conclusions comparing the results reported by different authors because of the inhomogeneous patient populations, but some studies have shown that the actuarial survival rates of patients with colorectal liver metastases undergoing cryoablation are similar to, if not better than, those of historical controls for standard resection (12). It is not certain that cryoablation is equivalent to surgical resection, but rather that this technique is an alternative option for patients with tumor which is considered unresectable. Now, cryosurgery appears the only therapy other than standard resection that has been demonstrated to provide long-term survival for patients with colorectal liver metastases (30).
In some cases, cryosurgery in combination with resection is the choice of treatment. If tumors are present near the hepatic hilum or within both lobes of the liver, patients will often be deemed unresectable. The combination of cryoablation with a resective procedure permits the preservation of as much functioning hepatic tissue as possible, reducing the scope of resection, and could potentially result in long-term survival. For example, a patient with multiple lesions in the right lobe and a solitary lesion in the left lobe would not be a good candidate for either cryoablation alone or a standard resection. However, a right hepatic lobectomy in combination with cryoablation of the lesion in the left lobe may bring about satisfactory effects (31).
Other options for unresectable hepatic malignancies, such as radiofrequency or microwave ablation and percutaneous ethanol injections, have been used, but postoperative higher recurrence, painful procedure or need of multiple procedures limit their use.
Position of percutaneous cryosurgery
Open cryosurgery is still a more invasive therapy to the patient. With the advancement of imaging guidance and improvement of cryosurgical apparatus, percutaneous cryosurgery, a less invasive procedure, has been used for treatment of tumors. Our cases who received percutaneous cryosurgery had survival similar to that of chemotherapy or resection. The result is encouraging, if considering in terms of unresectability of tumor and therapy-associated modalities (29).
Similar to open cryosurgery, the main problem in the face of percutaneous cryosurgery is recurrence of disease. The liver is a main site of recurrence, and extrahepatic recurrence is mainly seen in the lungs. It is noted that according to our data, the overall recurrence rate was lower than 44% in a mean follow-up of 16 months reported by Adam et al. (21) and much lower than the 78% reported by Weaver et al. (9). Moreover, the recurrence rate at the cryosite, including at cryosite only as well as both cryosites and the remaining areas of liver, was only 15.3% for cases who had recurrence, and 6.4% for all cases. This is significantly lower than the 58.8% reported by Jungraithmayr et al. (16). We discovered that the higher recurrence rate was mainly seen in patients who received cryosurgery at an early period after starting this technique. It is evident that gathering experience is an important factor for an increased effect.
Factors influencing survival
Distribution of metastases, operation type, total number of metastases, number of cryotreated metastases, largest size of cryotreated metastasis, and pre- and postoperative CEA have been considered the possible factors influencing prognosis of patients with colorectal liver metastases.
Yan et al. (32) critically evaluated the prognostic determinants for disease-free survival (DFS) after cryoablation in135 patients who underwent cryoablation with or without resection. In multivariate analysis, resection plus cryoablation, ≤7 liver metastases and ≤3 cm cryotreated metastasis were independently associated with an improved cryosite DFS. Our study showed patients with lesions ≤3 cm had an increased survival rate compared with those with lesions >3 cm, with a median survival of 39 and 21 months, respectively. This may have been due to the larger tumor in the vicinity of large vessels and exposure to the heat-sink effect (29). The warming effect of blood flow can cause insufficient cryodestruction of the tumor. Pearson et al. (33) reported that 66.7% of local recurrence occurred directly near the vena cava or a large vessel.
Contrast to Yan’s study, our study showed no significant correlation between the number of metastases and survival. This may be related with the difference between percutaneous and open approaches of cryoablation. In our study, patients with tumor in the right hepatic lobe have higher survival compared with those with left lobe or bilateral tumors, which may be due to the latter location is closer to large vessels.
Our study also showed a correlation between poor survival and CEA level >100 ng/dL, with a median survival of 18 mo, which is lesser than the 38 mo median survival for patients with lower CEA (P<0.01).The result is consistent with the report by Weaver (9,19) who showed that patients with CEA >100 ng/dL prior to cryosurgery had only 10 mo median survival, while the median survival of patients with CEA lower than that level was 17-19 months (29). Preoperative CEA of ≤5 ng/mL was independently associated with an improved overall and extrahepatic disease-free survival (34). The poor outcome of patients with higher CEA may be related to the biological behavior of CEA-secreting tumors or spreading of tumor.
According to the results of our study, the possibility of repeat percutaneous cryosurgery may be a factor which brings about better survival and low recurrence. Patients who received two or three cryosurgery procedures had longer survival. In contrast to operative cryosurgery, percutaneous cryosurgery may be performed many times because of its convenience and low intervention. As a result, the recurrence in liver and extrahepatic metastases may be simultaneously treated. In this series, there were 12 and 6 patients, respectively, with lung and pancreas.
Compared with hepatocellular carcinoma, patients with colorectal liver metastases appear to have a trend toward increased survival after cryosurgery. The reason may be due to the associated liver cirrhosis and reduced liver reserve in most of the former case (18).
Most metastases from noncolorectal primaries are unlikely to benefit from local therapies because the majority of these patients almost certainly have extra-hepatic disease at the time of the diagnosis of the liver disease. Therefore, local control of the liver disease is unlikely to significantly affect the clinical course and outcome. There are a few key exceptions. These include breast cancer, which can have a relatively indolent natural history in some patients such that local control of hepatic metastases will have a positive impact on the ultimate outcome (25,35). Neuroendocrine tumors and renal cell carcinoma can have some benefits in the cases of isolated metastases (36). For some metastases from other primary sites, cryoablation should be considered based on case-by-case basis.
Ways to increase the cryoablative effect
There are few data on ways to increase the cryoablative rates for colorectal liver metastases. However, in term of actual effect to remove cancerous tissue, cryosurgery is similar to resection, the modalities aiming to increase the resection effect are presumed to be adaptable for cryosurgery. Moreover, in contrast to primary liver cancer, which is often associated with liver cirrhosis and poor hepatic reserve function, colorectal liver metastases have a background of a relatively normal liver, therefore, the following adjunctive modalities can be safely applied.
Pre-cryoablative and post-cryoablative chemotherapy
In contrast to primary liver carcinoma, colorectal liver metastases are relatively sensitive to chemotherapy. It is known that the combination of resection with chemotherapy (neoadjuvant and adjuvant therapy) has survival benefits for patients with colorectal cancer or colorectal liver metastases. In 15-30% of the patients with colorectal liver metastases which initially was considered unresectable, these metastases were given macroscopically curative resection after chemotherapy (37,38). Therefore, the cryosurgery in combination with chemotherapy can be considered.
Portal vein embolisation
Resection of liver metastases may lead to severe postoperative liver failure if the functional reserve of the remaining liver remnant is too small (37). To overcome this problem, preoperative portal vein embolisation (PVE) can be considered. Portal vein occlusion leads to atrophy of the homolateral liver lobe and compensatory hypertrophy of the contralateral liver lobe (39,40). Hepatocyte regeneration starts within 1 day after PVE and reaches a peak at 12-14 days (41,42). The increase in volume of the remnant liver ranges from 7% to 27% (median 12%) after PVE (43). In this way, the functional reserve of the liver can be increased within 2-4 weeks.
From 1986 to 2000, 16 series had been published about PVE including 409 patients (44). The complication rate of PVE ranged from 0% to 10% (45). The 5-year survival rate was significantly higher in the PVE group than that in the transarterial embolization group (71.9% vs. 45.6%). Therefore, it is assumed that PVE before cryosurgery can be helpful for treatment of larger tumor of colorectal liver metastases as well.
Staged cryosurgery
A strategy has been suggested that in patients with multi-nodular metastases of the liver, staged liver resection can be performed as an alternative for conservative treatment. During the first operation, the larger tumors are resected. After operation, the liver remnant regenerates and is accompanied by an increase of the reserve capacity of the liver. During this period, systemic chemotherapy is given to hamper outgrowth of metastases. At a later stage, as the functional capacity has been restored, a second-stage curative resection of the residual liver metastases is performed. Whether this strategy can be used for cryosurgery is noteworthy.
Regional chemotherapy and isolated liver perfusion
The use of hepatic arterial infusion chemotherapy is based on the principle that the regional administration of certain drugs can lead to higher drug concentrations within the tumor with less systemic side effects. The hepatic arterial chemotherapy is often used for treatment of colorectal metastases, but controlled studies fail to show a significant benefit in overall survival.
To increase the response rates of chemotherapy, isolated hepatic perfusion (IHP) was applied. Aigner et al. (46) treated 29 patients with colorectal metastases with IHP using 5-FU under hyperthermic condition, and the median survival was 8 months. Vahrmeijer et al. found an overall response rate of 29% and a median survival of 19 months in 24 patients treated with IHP using melphalan in patients with colorectal metastases (47,48). The addition of tumor necrosis factor (TNF) to the perfusate may improve the response rate (49).
Molecular targeted therapy
The newer targeted therapies, such as bevacizumab and cetuximab, alone and in combination with chemotherapy are used for colorectal liver metastases. The addition of bevacizumab/cetuximab to fluoropyrimidine-based chemotherapy, with or without irinotecan or oxaliplatin, in both the first- and second-line treatment of metastatic colorectal cancer, significantly increased the median progression-free survival or time to disease progression in most randomized controlled trials (50-53).
Conclusions
Cryosurgery is feasible and safe for treatment of colorectal liver metastases. The technique is primarily palliative but may provide a possibility of cure in selected patients. Advances in instrumentation and intra-procedural imaging technique are making cryosurgery a viable surgical therapeutic alternative in the management of patients with unresectable colorectal liver metastases. Percutaneous cryosurgery can yield effects similar to that of open cryosurgery in hands of experienced surgeons.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Netherlands Cancer Registry. Incidence of Cancer in The Netherlands, Sixth report 1994.
- Gilbert HA, Kagan AR. Fundamental Aspects of Metastases. In: Weiss L. eds. Metastases: Incidence, Detection and Valuation without Histologic Confirmation. Amsterdam: Elsevier, 1976:385-405.
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6. [PubMed]
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. [PubMed]
- Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford) 2010;12:227-38. [PubMed]
- Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg 2002;89:1396-401. [PubMed]
- Ruers TJ, Jager GJ, Wobbes T. Cryosurgery for colorectal liver metastases. Semin Oncol 2000;27:120-5. [PubMed]
- Ruers TJ, Joosten J, Jager GJ, et al. Long-term results of treating hepatic colorectal metastases with cryosurgery. Br J Surg 2001;88:844-9. [PubMed]
- Weaver ML, Atkinson D, Zemel R. Hepatic cryosurgery in treating colorectal metastases. Cancer 1995;76:210-4. [PubMed]
- Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193-201. [PubMed]
- Wallace JR, Christians KK, Quiroz FA, et al. Ablation of liver metastasis: is preoperative imaging sufficiently accurate? J Gastrointest Surg 2001;5:98-107. [PubMed]
- Cha C, Lee FT Jr, Rikkers LF, et al. Rationale for the combination of cryoablation with surgical resection of hepatic tumors. J Gastrointest Surg 2001;5:206-13. [PubMed]
- Bilchik AJ, Wood TF, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 2000;135:657-62; discussion 662-4. [PubMed]
- Goering JD, Mahvi DM, Niederhuber JE, et al. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg 2002;183:384-9. [PubMed]
- Kerkar S, Carlin AM, Sohn RL, et al. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery 2004;136:770-9. [PubMed]
- Jungraithmayr W, Burger D, Olschewski M, et al. Cryoablation of malignant liver tumors: results of a single center study. Hepatobiliary Pancreat Dis Int 2005;4:554-60. [PubMed]
- Onik G, Rubinsky B, Zemel R, et al. Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer 1991;67:901-7. [PubMed]
- Ravikumar TS, Kane R, Cady B, et al. A 5-year study of cryosurgery in the treatment of liver tumors. Arch Surg 1991;126:1520-3; discussion 1523-4. [PubMed]
- Weaver ML, Ashton JG, Zemel R. Treatment of colorectal liver metastases by cryotherapy. Semin Surg Oncol 1998;14:163-70. [PubMed]
- Seifert JK, Morris DL. Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg 1998;228:201-8. [PubMed]
- Adam R, Akpinar E, Johann M, et al. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg 1997;225:39-8; discussion 48-50.
- Brooks AJ, Wang F, Alfredson M, et al. Synchronous liver resection and cryotherapy for colorectal metastases: survival analysis. Surgeon 2005;3:265-8. [PubMed]
- Onik GM, Atkinson D, Zemel R, et al. Cryosurgery of liver cancer. Semin Surg Oncol 1993;9:309-17. [PubMed]
- Seifert JK, Heintz A, Junginger T. Cryotherapy for primary and secondary liver tumours. Zentralbl Chir 2002;127:275-81. [PubMed]
- Seifert JK, Junginger T. Cryotherapy for liver tumors: current status, perspectives, clinical results, and review of literature. Technol Cancer Res Treat 2004;3:151-63. [PubMed]
- Seifert JK, Springer A, Baier P, et al. Liver resection or cryotherapy for colorectal liver metastases: a prospective case control study. Int J Colorectal Dis 2005;20:507-20. [PubMed]
- Huang A, McCall JM, Weston MD, et al. Phase I study of percutaneous cryotherapy for colorectal liver metastasis. Br J Surg 2002;89:303-10. [PubMed]
- Mala T, Edwin B, Mathisen Ø, et al. Cryoablation of colorectal liver metastases: minimally invasive tumour control. Scand J Gastroenterol 2004;39:571-8. [PubMed]
- Mumtaz K, Majid S, Shah H, et al. Prevalence of gastric varices and results of sclerotherapy with N-butyl 2 cyanoacrylate for controlling acute gastric variceal bleeding. World J Gastroenterol 2007;13:1247-51. [PubMed]
- McWilliams JP, Yamamoto S, Raman SS, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol 2010;21:S204-13. [PubMed]
- Tatli S, Acar M, Tuncali K, et al. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol 2010;16:90-5. [PubMed]
- Martin RC. Hepatic tumor ablation: cryo versus radiofrequency, which is better? Am Surg 2006;72:391-2. [PubMed]
- Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999;178:592-9. [PubMed]
- Weiss MJ, D’Angelica MI. Patient selection for hepatic resection for metastatic colorectal cancer. J Gastrointest Oncol 2012;3:3-10. [PubMed]
- Martínez C J, Jarufe CN, González DR, et al. Current therapeutic options for liver metastasis. Rev Med Chil 2008;136:376-84. [PubMed]
- Kvols LK, Turaga KK, Strosberg J, et al. Role of interventional radiology in the treatment of patients with neuroendocrine metastases in the liver. J Natl Compr Canc Netw 2009;7:765-72. [PubMed]
- Bertheault-Cvitkovic F, Jami A, Ithzaki M, et al. Biweekly intensified ambulatory chronomodulated chemotherapy with oxaliplatin, fluorouracil, and leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 1996;14:2950-8. [PubMed]
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 1997;350:681-6. [PubMed]
- Bax HR, Mansens BJ, Schalm L. Atrophy of the liver after occlusion of the bile ducts or portal vein and compensatory hypertrophy of the unoccluded portion its clinical importance. Gastroenterology 1956;31:131-55. [PubMed]
- Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. [PubMed]
- Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993;18:79-85. [PubMed]
- Nagino M, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 1995;21:434-9. [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165-75. [PubMed]
- Seo DD, Lee HC, Jang MK, et al. Preoperative portal vein embolization and surgical resection in patients with hepatocellular carcinoma and small future liver remnant volume: comparison with transarterial chemoembolization. Ann Surg Oncol 2007;14:3501-9. [PubMed]
- Aigner KR, Walther H, Tonn JC, et al. Isolated liver perfusion in advanced metastases of colorectal cancers. Onkologie 1984;7:13-21. [PubMed]
- Vahrmeijer AL, van Dierendonck JH, Schutrups J, et al. Potentiation of the cytostatic effect of melphalan on colorectal cancer hepatic metastases by infusion of buthionine sulfoximine (BSO) in the rat: enhanced tumor glutathione depletion by infusion of BSO in the hepatic artery. Cancer Chemother Pharmacol 1999;44:111-6. [PubMed]
- Vahrmeijer AL, van Dierendonck JH, Keizer HJ, et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer 2000;82:1539-46. [PubMed]
- Alexander HR Jr, Bartlett DL, Libutti SK. Current status of isolated hepatic perfusion with or without tumor necrosis factor for the treatment of unresectable cancers confined to liver. Oncologist 2000;5:416-24. [PubMed]
- Board RE, Valle JW. Metastatic colorectal cancer: current systemic treatment options. Drugs 2007;67:1851-67. [PubMed]
- Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007;110:2761-7. [PubMed]
- McCormack PL, Keam SJ. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs 2008;68:487-506. [PubMed]
- Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734-8. [PubMed]