
Invisible and intangible illness: a qualitative interview study of patients’ experiences and understandings of conservatively managed end-stage kidney disease
Introduction
Over the last decade the number of people dying from chronic kidney disease has increased by almost one third (25.5–31.4%); this equates to approximately 1.2 million people dying each year from kidney disease globally (1). There has also been a marked shift towards deaths at older ages since the 1990s (1), and the end-stage kidney disease population is increasingly elderly and co-morbid (2).
For older adults facing a decision regarding renal replacement therapy, although survival in receipt of dialysis will most likely be longer, the burden of accessing dialysis may outweigh the survival benefit for some patients (3). Dialysis impacts heavily on quality of life requiring frequent hospital attendance (86% receive haemodialysis in hospital, 14% at home), usually 3 times per week (4). Conservative kidney management is considered a viable and beneficial option for older adults with comorbidities, particularly ischemic heart disease, for whom survival benefit of dialysis may be negligible (5-7). Patients receiving conservative management spend less time having treatment, and are less likely to require hospital admissions or die in hospital (8). However, access to and awareness of conservative management for older adults varies nationally, and depends partly on the degree to which conservative management is an established pathway within each unit (9,10). Such pathways include careful monitoring, interventions to delay progression of kidney disease, and management of uraemia and burdensome symptoms including, but not limited to: fatigue; anorexia; nausea and vomiting; pain and pruritis (11,12). People with conservatively managed end-stage kidney disease have considerable symptom control needs, similar to advanced cancer populations (12). Longitudinal research has shown a sharp increase in symptom distress and health related concerns for patients with conservatively managed kidney disease in the last 2 months of life (13).
The impact of chronic illness or long-term conditions upon the individual and those close to them has long been recognised. The ‘biographical disruption’ experienced often requires a fundamental rethinking of one’s identity, including plans and expectations (14). Chronic illness is also associated with loss of self, or former self, and suffering, often resulting in withdrawal and isolation (15). The coping strategies that individuals develop, however, are influenced by their perceptions and interpretations of their illness (16).
Research has highlighted the physical, social and psychological impact of kidney disease upon individuals (17-19), but has focused primarily on those in receipt of dialysis or transplant. The limited research into the experiences of those in receipt of conservative kidney management has focused on reasons for choosing that pathway (20-24), including communication and information provision, rather than the impact and understanding of their illness thereafter. This study therefore aimed to explore the experiences of older adults living with kidney disease that was being managed conservatively to examine: the impact of their illness, including the impact over time; and their understanding of the illness to inform clinical practice and policy.
Methods
Design
Secondary analysis of qualitative interview data. According to the typology of secondary analyses, this study represents a ‘supplementary analysis’ whereby the analysis focuses on emergent themes which were not addressed, or only partially addressed, by the primary analysis (25).
Setting
Participants were recruited from three renal units across South London and the South East of England, as part of a larger longitudinal study mixed methods (13). Participation in the larger study included consent for access to medical records, sharing of demographic information, a longitudinal quantitative survey [data from which are reported elsewhere (13)], and a single in depth qualitative interview undertaken in 2007 (secondary analysis of these interview data are reported in the current paper).
Governance
Ethical approval for the study was received by King’s College Hospital Research Ethics Committee (COREC: 04/03/092). Local R&D and ethical approvals were acquired at each site. As the supplementary analysis of the data undertaken fell within the broad aims and objectives of the original study, no further approvals were required.
Sampling
Participants were purposively sampled for diversity by: age; co-morbidity (assessed using the Charlson Comorbidity Index (26) modified for renal patients which excludes renal diagnosis and age from the scoring (27) and functional status [assessed using the Karnofsky Performance Status (28)].
Inclusion criteria
Adults (>18 years) under the care of nephrologists with stage 5 chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) ≤15 mL/minute and a confirmed (by nephrologist) decision for conservative management (management without dialysis or renal replacement therapy).
Exclusion criteria
Lacking capacity to give consent to participate in the study.
Recruitment
Potentially eligible patients were identified by their clinicians, after assessment of their capacity at a clinic appointment, and given verbal and written information about the study. Clinicians sought permission for the researcher (FE Murtagh) to make contact, and the preferred means of that contact. At least 24 hours after this first approach, the researcher contacted those who had expressed an interest in participation. Only one of the participants who was approached declined interview, as they felt too unwell. For all others a further appointment was then made, at the patient’s preferred location, at which written informed consent was taken to participate in the study, and the interview conducted. Recruitment continued until data saturation was achieved and no new themes, related to the aims and objectives of the original study, were emerging from subsequent interviews.
Interviews
Qualitative interviews were chosen to allow in-depth exploration of the illness experience for older adults living with kidney disease. A topic guide was developed, and piloted with three participants before being finalised (pilot interview data not included). In-depth interviews were conducted in the participant’s preferred location (all in their usual place of residence), to explore their experiences of the illness, the impact of their illness and symptoms, and their involvement in decision-making. For 13 interviews, only the patient was present in the room for the interview; the remaining seven participants chose for their spouse or a relative to remain in the room. All interviews were conducted by FE Murtagh, a palliative care clinician. She was not known to the participants, was not involved in their care, and was introduced as a researcher. Interviews were audio recorded, transcribed verbatim and anonymised. Field notes were also taken at the time of the interview and immediately after, to capture contextual factors. No repeat interviews were undertaken, and transcripts were not returned to participants for comments.
Analysis
Interviews were transcribed verbatim, and transcripts were anonymised with allocation of culturally appropriate pseudonyms. The study was positioned within a subtle realist paradigm which assumes that there is an objective reality, but that it can only be understood through an individual’s perspective of it (29). Interviews were analysed inductively using thematic analysis (30,31), which involved six steps: familiarisation; coding; searching; reviewing; and defining themes; and reporting. This method was chosen as it offers the researcher an accessible and theoretically flexible approach to analysing qualitative data, shaped to the research question and epistemology (32). The researcher (FE Murtagh) had not previously undertaken in-depth interviews, although had completed an intensive training course at the National Centre for Social Research to develop these skills. Early transcripts were critically reviewed by an independent experienced qualitative interviewer, to provide feedback and to refine techniques. Analysis was further discussed and refined by FE Murtagh, K Bristowe and LE Selman. Analysis was supported by QSR NVivo qualitative data analysis software.
Results
Participants
Twenty older people (>65) living with kidney disease were interviewed. The participants: had a median age of 82 years (range, 69–95 years); 11 were men and 9 women; 18 were of white ethnicity, one black Caribbean, and one other ethnicity (ethnicity was categorised according to those used in UK Renal Registry report); 8 were married, 9 were widowed, and 3 were single; 10 were living alone, 7 with their spouse, 2 with family (both with daughters), and 1 in a nursing home (see Table 1). Median Karnofsky Performance Status score was 60% (range, 40–80%) (28), and median modified Charlson Comorbidity Index score (which excludes renal diagnosis and age from the scoring) was 4 (range, 4–8) (27). The duration of the interviews ranged from 38 to 74 minutes.
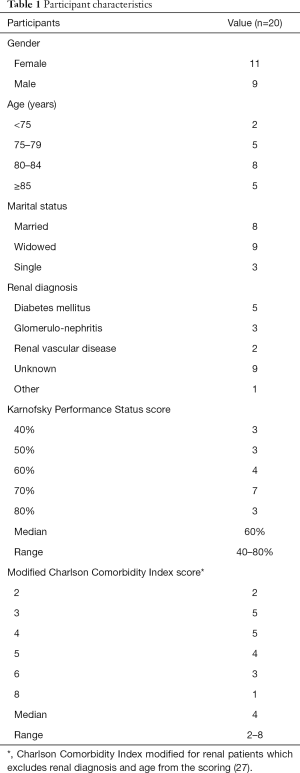
Full table
Findings
Participants described the challenges of living with kidney disease, particularly in terms of its hidden nature. They described it as invisible and intangible, struggling to attribute symptoms to the illness, whilst also feeling disconnected from it. This combination of invisibility and intangibility, alongside the unpredictable nature of the illness, impacted heavily on individuals’ quality of life.
Invisibility
In expressing their experience of the illness, kidney disease was described by participants as a ‘hidden’ or ‘invisible’ illness. Participants made reference, with varying degrees of detail, to the perception that they were in the grip of something unseen. Jack, for instance, gave a graphic description of his kidney disease as an invisible illness.
“Like there’s something bubbling along in your blood that you don’t know quite what it will do, and you can’t see it.” (Jack, 84 years old).
Several participants contrasted kidney disease with other illnesses that they had experienced, pointing out the lack of form or identity for themselves or others to grasp. Jean described it as something hidden from her, with ‘nothing to show for it’, contrasting it with her (severe) arthritis that was visible in her hands and knees.
“There isn’t anything like I could say ‘this is the kidney problem doing this’—it is all a bit more silent, going along there but without me really knowing, brewing away but nothing to see really…I mean, they tell you the kidney isn’t good, but well…how do I know? There’s nothing to show for it, is there?..I’m not to know, am I? It’s kind of hidden, I don’t know it’s there.” (Jean, 81 years old).
The lack of form and invisibility of kidney disease was further underpinned by descriptions of the illness almost as a ‘spectre’ or ‘being’, which would ‘creep up on you’, giving it agency and control over individuals’ actions.
“Yes, and you don’t notice it, for a while you don’t really notice that you’re giving things up, it kind of creeps up on you, and then suddenly you do things like, a major one was why the hell get dressed when it takes me an hour to get dressed, why not just stay in my dressing gown.” (Charles, 77 years old).
The unpredictability and lack of control associated with the illness was particularly challenging for George, who described being ‘in the hands of a mystery’.
“It’s like it’s some vis…invisible thing going on, and we don’t know. I mean they know the kidneys is bad, don’t they, but why does it go up and down and why does it, it causes all these problems? We’re in the hands of a mystery, I think.” (George, 95 years old).
Intangibility
A further extension of the invisibility of the kidney disease involved challenges in attributing symptoms to the illness. Participants described not knowing whether it was the illness causing symptoms or problems, whether they were age-related, or caused by other co-morbid conditions. This contributed to the sense of intangibility of the illness, as described by Grace.
“But I don’t know if that be kidney or not. My heart’s bad and my breathing sometimes, and my legs play me bad at night. It could be all that, or something’ else, I don’t know (laughs).” (Grace, 73 years old).
Other participants were also uncertain, not in describing the symptoms, but in knowing what had caused them. Some, such as Harold, who was 82 years old, included their age as a possible reason for symptoms or problems.
“Another thing is I find I get very tired these days, I don’t know whether it’s that (meaning his kidney disease) or old age, I mean, you know, I’ve never been this age before.” (Harold, 82 years old).
This uncertainty in attribution contributed to participants’ sense of a loss of control. Not only could they do little about the effects of the illness, but neither could participants explain what was causing them. This in turn influenced their feelings towards their illness and their perception of other people’s attitudes towards them.
“With my kidneys…it’s, well…hard to say, I just feel tired and no energy, and generally not myself, all vague and sounds like I’m just…complaining or something. Making a fuss about nothing (laughs)”. (Donald, 89 years old).
The loss of control experienced by individuals was exacerbated by the unpredictable nature of kidney disease, which severely impacted on their ability to complete or plan day-to-day activities, negatively affecting their quality of life. Specifically, some individuals described not being able to predict a deterioration from one day to the next.
“The worst of it is, I never quite know how I’m going to be. Sometimes I can get up and I’ll be fine, it will be a good day, and that will carry on for a bit, and then out the blue, it all goes, like I’ve run out of petrol or something, and there’s nothing in the tank. I can feel unwell for days then, but I never really know why it’s like that, this way, that way, can’t tell how I’ll be.” (Florence, 82 years old).
The physical impact of kidney disease was also described as vague or intangible, beyond tiredness or fatigue, almost as a weight, holding you down, suspending your ability to carry out activities.
“It’s hard to describe….It’s rather like someone put lead weights on your shoulders or something, you just can’t get up and get going. It runs through everything, all the time. Sometimes it’s not so bad for a few weeks, and I think maybe I can do a few things, then suddenly it’s back again, and I can’t get going…” (Betty, 79 years old).
This sense of being held down was also described as a loss of ‘get up and go’, lack of ‘impetus’, feeling they had no ‘fuel in the tank’, or ‘lifelessness’. This effect was pervasive, impacting on all aspects of daily living, and the burden was exacerbated due to the unpredictability of the illness.
“The energy levels too, it was just steadily getting worse. Harder to do things, like I said, it’s hard to explain, but it was very difficult to say ‘I’m going to do this,’ and then do it. Normally you don’t think of it, you just get on and do, don’t you, but here I was, spending 20, 30 minutes saying ‘I’m going to do’ and then still not doing, oh, even the simplest of things.” (Franklyn, 86 years old).
For many the stark contrast between their life now and prior to their illness was overwhelming, particularly due to the often sudden deterioration affecting all aspects of their life.
“It has affected me terrible. Yes, you know. Nothing is the same…I don’t have the strength or the energy any more, it makes me feel useless, I couldn’t do what I wanted to do any more, but, there you are, you know what I mean, it’s so bad, I mean now I can’t even write, I can’t write properly….I don’t know. It just seems to have struck me right down, it just came down like a ton of bricks, you know, it came right down on top of me.” (Dorothy, 84 years old).
Disconnectedness
The invisible nature of the disease was also associated with a feeling of disconnectedness from it. Donald felt it was professionals who were telling him of his illness, but that this did not correspond to the evidence from his own senses; as he said, ‘can’t see it, don’t feel it’.
“I dunno, dunno as it has affected me. Can’t pin anything down, that’s what I said to Dr. (GP’s name). Can’t see it, don’t feel it. Wouldn’t know about it, but for them….It’s like someone tells you…your kidneys are bad…but it’s nothing to do with you, you have to believe them.”
And he later went on to say:
“If it’s my heart, then I might get pain, alright I don’t know if it’s indigestion or what, but I get a pain. With my kidneys…it’s, well…hard to say, I just feel tired and no energy.” (Donald, 89 years old).
Others felt unable to pinpoint the illness as a specific cause, and relied on professionals to tell them what was happening.
“But it’s hard, if you have a pain in your back, you know it’s your back, if you have a muscle go in your hand, it’s in your hand. But you don’t know what your kidneys are doing do you, so it’s hard to say anything much about them, you have to go on what they (clinicians) say to you, with them bloods and that what they take.” (Alice, 82 years old).
The invisibility and intangibility of the illness fueled the sense of disconnectedness from it. Collectively, these resulted in challenges in attributing symptoms to the kidney disease, reduced ability to plan and engage in activities, and poor quality of life.
Discussion
This is one of relatively few studies to examine the experience of older adults living with end-stage kidney disease in receipt of conservative kidney management. Participants described the invisibility and intangibility of kidney disease, the challenges of attributing symptoms to the disease, and the unpredictable, and often sudden, nature of deterioration over time. They described a spectre-like presence or force, sapping their energy and holding them down. For some, it was hard to differentiate the symptoms of the illness from characteristics of ageing, resulting in challenges in illness attribution, and a sense of disconnectedness from the illness.
Challenges in illness attribution have been recognised in other invisible illnesses including osteoporosis, where lack of symptoms created doubt and ambivalence regarding diagnosis (33), SLE (systemic lupus erythematosus) where invisibility had detrimental effects on adjustment (34), and COPD (chronic obstructive pulmonary disease) where invisible signs of deterioration were not recognised by professionals (35). Indeed, as in the present study, where the condition is perceived as abstract, this impacts on the ability to make sense of and cope with illness (16). The invisibility and intangibility of kidney disease resulted in participants’ lack of understanding of their illness, uncertainty, and a resultant inability to attribute symptoms and concerns to the illness. This was associated with a loss of self (15) to a liminal state between illness and wellness (36,37), and a lack of access to the sick role (38), and the associated rights and obligations. The challenges of managing uncertainty in the context of advanced illness have also been recognised in cancer, heart failure, COPD and liver disease. Better understanding of the patient’s goals and temporal focus (present or future), alongside exploration of their information needs and engagement with illness is critical to enable open and honest discussions about future care and preferences (39). Indeed, in the present study although participants spoke about deterioration more broadly and referred implicitly to their deterioration and death, very few spoke overtly about end of life care or used terms of this type.
Previous studies have recognised the variation in understanding of the nature and purpose of conservative kidney management across the UK (9,10). This disparity often appears to stem from inadequate communication about: the distinction between conservative care and more interventional approaches; prognosis; and the palliative nature of conservative care (40). Indeed nephrologists describe a reluctance to open discussions about prognosis with their patients until a deterioration is evident, due to long established relationships with patients and to avoid causing distress (41,42). The present study supports a need for improved communication, education and support regarding conservative care pathways, at the point of critical decisions, including: expectations with regard to symptoms and impact of the disease upon the lifeworld, alongside consideration of the burden of dialysis. Research has highlighted that nephrologists underestimate the presence and severity of symptoms for haemodialysis patients (43). It is critical that regular assessment of conservatively managed patients includes psychological, social and spiritual domains, alongside physical concerns, to ensure that: symptoms and concerns are recognised and addressed. Moreover, it is essential that adjunct services to provide psychological, social and spiritual support are equally available to conservatively managed patients who may rarely attend the hospital, as they are for those attending regularly for dialysis.
Strengths and limitations
This study has both strengths and limitations. The study sample represents a maximum variation sample, with regard to age, living situation, levels of co-morbidity and functional status, which adequately reflects the variability within this population. As this is a qualitative study, it does not seek for findings to be generalisable, however findings would be transferable to other sites with aging end-stage kidney disease populations. Most participants in the study were of white ethnicity, reflecting the UK population and the catchment population for the three renal units. Further interviews with individuals from Black, Asian and minority ethnic communities would have increased the transferability of these findings. Seven individuals chose to undertake interviews with a family member present in the room. Whilst they did not participate in the interviews, it is possible that their presence may have influenced some responses to questions. However, we chose to undertake interviews in line with the preferences of each individual, some of whom preferred to have someone else present for support. The data for this study were originally collected in 2007, since which time conservative management has become a more accepted component of renal care. However there is recent evidence of significant variability in service delivery across renal units (44), and there continues to be a lack of research exploring the lived experiences of people living with conservatively managed kidney disease. Our findings are therefore still novel and highly relevant. As a secondary (supplementary) analysis of an existing dataset, it was not possible to undertake an iterative process of recruitment and analysis, and therefore data saturation was more challenging to assess. The original study addressed wider aims related to the experience of conservative kidney care, with themes across broader domains. Primary research focusing specifically on the invisibility and intangibility of kidney disease may extend our findings. Given the social and psychological impact of end-stage kidney disease beyond the individual living with the disease, further interviews with informal caregivers, and healthcare professionals delivering conservative kidney care, would also be beneficial, to further examine the invisibility and intangibility of this condition. In addition, longitudinal qualitative work with diverse patient groups would enable further exploration of how perspectives and understandings of conservatively managed kidney disease evolve over time, to inform intervention development.
Conclusions
This original paper describes, for the first time, the profound invisibility and intangibility of the illness perceived by those people with end-stage kidney disease managed conservatively, without dialysis. Challenges in differentiating the burdensome symptoms of end-stage kidney disease from normal aging are shared with other illnesses, but the profound intangibility causes major challenges to individuals’ sense of self and obstructs development of realistic hopes and aspirations (45). A sharp increase in symptom distress and health concerns for those with conservatively managed kidney disease may suggest a marked deterioration or change in phase of illness, demonstrating the importance of recognition and management of symptoms (13). However, differences between the patient’s interpretation of this intangible illness and its effects on their life, and the professional’s biomedical symptom focused approach, may preclude meaningful discussions about problems, concerns and future care (46). Clinical services need to recognise the invisibility, intangibility, and related sense of disconnectedness, and address this through specific interventions focused on improving clinical assessment and communication, education, peer support, and professional support.
Acknowledgements
This study was funded by the Guy’s and St. Thomas’ Charity.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval for the study was received by King’s College Hospital Research Ethics Committee (COREC: 04/03/092). Written informed consent was obtained from all participants.
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. [Crossref] [PubMed]
- UK Renal Registry. UK Renal Registry Report. Bristol, UK Renal Registry 2006.
- Morton RL, Snelling P, Webster AC, et al. Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 2012;184:E277-83. [Crossref] [PubMed]
- Byrne C, Caskey F, Castledine C, et al. UK Renal Registry 19th Annual Report of the Renal Association. Available online: https://www.renalreg.org/wp-content/uploads/2017/09/19th-Annual-Report_web_book.pdf
- Murtagh FE, Marsh JE, Donohoe P, et al. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007;22:1955-62. [Crossref] [PubMed]
- Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011;26:1608-14. [Crossref] [PubMed]
- Verberne WR, Geers AB, Jellema WT, et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. CJASN 2016;11:633-40. [Crossref] [PubMed]
- Hussain JA, Mooney A, Russon L. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 2013;27:829-39. [Crossref] [PubMed]
- Gessert CE, Haller IV, Johnson BP. Regional variation in care at the end of life: discontinuation of dialysis. BMC Geriatr 2013;13:39. [Crossref] [PubMed]
- Morton RL, Webster AC, McGeechan K, et al. Conservative Management and End-of-Life Care in an Australian Cohort with ESRD. CJASN 2016;11:2195-203. [Crossref] [PubMed]
- Murtagh FE, Burns A, Moranne O, et al. Supportive Care: Comprehensive Conservative Care in End-Stage Kidney Disease. Clin J Am Soc Nephrol 2016;11:1909-14. [Crossref] [PubMed]
- Murtagh FE, Addington-Hall JM, Edmonds PM, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 2007;10:1266-76. [Crossref] [PubMed]
- Murtagh FE, Sheerin NS, Addington-Hall J, et al. Trajectories of illness in stage 5 chronic kidney disease: a longitudinal study of patient symptoms and concerns in the last year of life. Clin J Am Soc Nephrol 2011;6:1580-90. [Crossref] [PubMed]
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. [Crossref] [PubMed]
- Charmaz K. Loss of self: a fundamental form of suffering in the chronically ill. Sociol Health Illn 1983;5:168-95. [Crossref] [PubMed]
- Leventhal H, Brissette I, Levethal EA. The common-sense model of self-regulation of health and illness. In: Cameron L, Leventhal H. Editors. The self-regulation of health and illness behaviour. London: Routledge, 2003:42-65.
- Bristowe K, Horsley H, Shepherd K, et al. Thinking Ahead: The Importance of Early Advance Care Planning for Haemodialysis Patients. Palliat Med 2015;29:443-50. [Crossref] [PubMed]
- Lowney AC, Myles HT, Bristowe K, et al. Understanding What Influences the Health-Related Quality of Life of Hemodialysis Patients: A Collaborative Study in England and Ireland. J Pain Symptom Manage 2015;50:778-85. [Crossref] [PubMed]
- Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage 2010;39:477-85. [Crossref] [PubMed]
- Visser A, Dijkstra GJ, Kuiper D, et al. Accepting or declining dialysis: considerations taken into account by elderly patients with end-stage renal disease. J Nephrol 2009;22:794-9. [PubMed]
- Noble H, Meyer J, Bridges J, et al. Reasons Renal Patients Give for Deciding Not to Dialyze: A Prospective Qualitative Interview Study. Dial Transplant 2009;38:82-9. [Crossref]
- Johnston S, Noble H. Factors influencing patients with stage 5 chronic kidney disease to opt for conservative management: a practitioner research study. J Clin Nurs 2012;21:1215-22. [Crossref] [PubMed]
- Morton RL, Tong A, Howard K, et al. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010;340:c112. [Crossref] [PubMed]
- Seah AS, Tan F, Srinivas S, et al. Opting out of dialysis – Exploring patients' decisions to forego dialysis in favour of conservative non-dialytic management for end-stage renal disease. Health Expect 2015;18:1018-29. [Crossref] [PubMed]
- Heaton J. Secondary Analysis of Qualitative Data: An Overview. Hist Soz Forsch 2008;33:33-45.
- Charlson M, Szatrowski T, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol 2012;44:1813-23. [Crossref] [PubMed]
- Mor V, Laliberte L, Morris J, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002-7. [Crossref] [PubMed]
- Hammersley M. Challenging Relativism: The Problem of Assessment Criteria. Qual Inq 2009;15:3-29. [Crossref]
- Miles M, Huberman A. Qualitative Data Analysis. London: Sage, 1994.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. [Crossref]
- Bristowe K, Selman L, Murtagh F. Qualitative research methods in renal medicine: an introduction. Nephrol Dial Transplant 2015;30:1424-31. [Crossref] [PubMed]
- Weston JM, Norris EV, Clark EM. The invisible disease: making sense of an osteoporosis diagnosis in older age. Qual Health Res 2011;21:1692-704. [Crossref] [PubMed]
- Brennan KA, Creaven AM. Living with invisible illness: social support experiences of individuals with systemic lupus erythematosus. Qual Life Res 2016;25:1227-35. [Crossref] [PubMed]
- Cawley D, Billings J, Oliver D, et al. Potential triggers for the holistic assessment of people with severe chronic obstructive pulmonary disease: analysis of multiperspective, serial qualitative interviews. BMJ Support Palliat Care 2014;4:152-60. [Crossref] [PubMed]
- Brown B, Huszar K, Chapman R. 'Betwixt and between'; liminality in recovery stories from people with myalgic encephalomyelitis (ME) or chronic fatigue syndrome (CFS). Sociol Health Illn 2017;39:696-710. [Crossref] [PubMed]
- Turner V. The Ritual Process. Chicago: Aldine, 1969.
- Parsons T. Social Structure and Dynamic Process: The Case of Modern Medical Practice. Social System. London: Routledge and Kegan Paul Ltd., 1951.
- Etkind SN, Bristowe K, Bailey K, et al. How does uncertainty shape patient experience in advanced illness? A secondary analysis of qualitative data. Palliat Med 2017;31:171-80. [Crossref] [PubMed]
- Low J, Myers J, Smith G, et al. The experiences of close persons caring for people with chronic kidney disease stage 5 on conservative kidney management: contested discourses of ageing. Health (London) 2014;18:613-30. [Crossref] [PubMed]
- Lazenby S, Edwards A, Samuriwo R, et al. End-of-life care decisions for haemodialysis patients - 'We only tend to have that discussion with them when they start deteriorating'. Health Expect 2017;20:260-73. [Crossref] [PubMed]
- Schell JO, Patel UD, Steinhauser KE, et al. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis 2012;59:495-503. [Crossref] [PubMed]
- Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 2007;2:960-7. [Crossref] [PubMed]
- UK Renal Registry. Patient Reported Experience of Kidney Care in England and Wales 2017. Bristol, UK, 2018.
- Davison SN, Simpson C. Hope and advance care planning in patients with end stage renal disease: qualitative interview study. BMJ 2006;333:866. [Crossref] [PubMed]
- Stewart M, Brown J, Weston W, et al. Patient-centred medicine: transforming the clinical method. BMJ 1995;311:1580. [Crossref]


