
Cancer cachexia is defined by an ongoing loss of skeletal muscle mass
A view of cancer cachexia through a radiological lens
While experts agree that the loss of skeletal muscle is a defining feature of cancer cachexia (1), muscle has always been difficult to evaluate in a clinical setting other than by a purely functional or crude anthropometric approach. The 1980’s saw the initiation and validation of image-based modalities [computed tomography (CT) and magnetic resonance imaging (MRI)] for the quantification of skeletal muscle (2-4). The precision of measures of skeletal muscle cross-sectional area or volume with these approaches is of the order of 0.4% to 1.5% depending on the model or instrument used and image- or patient- or operator-related sources of variation (3). These methods have allowed us to assess the muscularity of different individuals, to relate muscle mass to disease specific outcomes, to define sarcopenia (severe muscle depletion) in quantitative terms, to detect catabolic loss or gain of muscle over time, to determine the behavior of specific individual muscles and to define the efficacy of different therapies developed for the treatment of muscle wasting. CT images acquired as part of standard cancer care were identified as a suitable substrate for these types of assessments in ~2008 (5) and have emerged as a particularly rich resource for the study of body composition in relation to oncological outcomes. This approach makes maximal use of existing information, has no incremental impact on the patient and has permitted countless retrospective and prospective studies. Images are particularly abundant in patients on systemic therapy treatment with palliative intent, and are ubiquitously used to plan cancer surgery. Computed tomography methods have been widely adopted by researchers, medical oncologists, surgeons, dietitians and palliative care physicians (6-8). Individual data sets with thousands of patients have started to appear in publications (9-11), and collectively tens of thousands of individuals have been assessed. Many authors have advocated for the extraction of the body composition data from clinical CT records (5,12,13). To date, this information is not yet part of standard radiology reporting, and is being conducted instead by researchers and a variety of health care professionals.
In 2007/2008 early reports showing association between sarcopenia or sarcopenic obesity and two cancer outcomes, chemotherapy-related toxicity and mortality began to emerge (14-16). In the subsequent decade owing to widespread adoption of CT assessments, over 950 publications have catalogued the body composition of different populations of cancer patients. Three radiologically-determined abnormalities, sarcopenia (severe muscle depletion), catabolic loss of muscle over time, and reduced muscle radiation attenuation associate with mortality, complications of cancer surgery, chemotherapy toxicity, physical functioning and quality of life. The reader is referred to the many reviews and meta-analyses of such work, which now increasingly are specific to tumor-site and treatment [e.g., (17)].
In general, cross-sectional analysis of single CT images, typically landmarked at the 3rd lumbar vertebra (L3) (Figure 1) is conducted. Muscle cross sectional area (cm2) in single axial images at this level were shown to have a good correlation with while body muscle volume (r2 =0.85) by Shen et al. (18). Whole body imaging is rare in clinical oncology; an approach using L1 has been suggested for patients with thoracic imaging only (19,20).
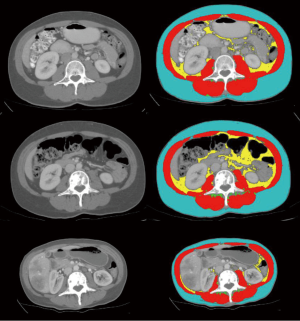
Diagnostic imaging allows detailed assessment of the individual cachexia trajectory. The person illustrated in Figure 1 had a diagnosis of metastatic breast cancer. Compared to time of diagnosis, she lost 10.5% of muscle by year 2 and 29.1% by year 3 (end of life). A transient gain of visceral fat (+93%) and subcutaneous fat (+8%) was seen at year 2, but by end of life 72% of baseline visceral fat and 62% of subcutaneous fat had been lost.
An international consensus of clinical experts (1) defined cancer cachexia as “…being characterized by loss of muscle, with or without loss of fat mass”. The concurrent loss of muscle and gain in fat of the patient in Figure 1 typify this statement. The eventual result of such changes, sarcopenic obesity, is not uncommon in patients with advanced cancer. Sarcopenia and muscle wasting are endemic in patients with advanced cancer in westernized countries, but the same populations are afflicted with epidemic obesity. Literature on sarcopenic obesity in patients with locally recurrent or metastatic cancers was recently summarized (21). The overall prevalence of sarcopenic obesity was 9.3% (range, 2.3–14.6%), and 24% (range, 5.9–39.2%) of patients with a BMI >30 kg/m2 were sarcopenic. As also summarized by these authors, sarcopenic obesity associates with exceptionally poor clinical outcomes, including complications of cancer surgery, chemotherapy toxicity and mortality.
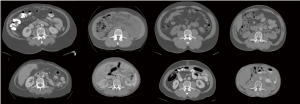
Each patient’s experience of cancer cachexia is uniquely defined by their pre-illness body habitus and their specific trajectory of loss (Figure 2). Levels of adipose tissue in subcutaneous and visceral compartments are highly variable among individuals, as is muscularity. Rate of muscle loss over time associates with mortality. For example, Blauwhoff-Buskermolen et al. (22) showed that in metastatic colon cancer, the patients with the highest rate of muscle loss (>9%) during a course of palliative chemotherapy, had the highest mortality.
Radiation attenuation is a second radiologic characteristic of muscle that is the subject of recent interest as it is independently related to mortality in melanoma, renal cell carcinoma, lung and gastrointestinal cancers (9,23-25). Considering the entire organ, any given skeletal muscle displays radiation attenuation between −190 to +150 HU. When muscle cross-sectional area and attenuation are reported in the literature, the most common practice is to use predefined HU ranges (5,26). Inter-muscular adipose tissue is separately segmented between −190 and −30 HU. The HU range used for muscle typically extends from −29 to +150 HU. The radiation attenuation of healthy young adults has a prominent peak around +50 and +30 HU is considered to be the lower bound of normal muscle. Older persons typically have a proportion of muscle considered to be of low radiation attenuation (−29 to +29 HU). For discussion of these thresholds, see Aubrey et al. (26).
The last 5 years have seen the appearance of multiple publications associating reduced muscle radiodensity (i.e., <25–30 HU) with mortality, and this effect is independent of other clinical covariates and also independent of sarcopenia in patients with solid tumors [e.g., (9,11,17,23)] as well as in hematological cancers (24).
Biological correlates of the radiological findings
Disruption of the anabolic: catabolic balance in skeletal muscle
For a recent review of this area, see Baracos et al. (27). Briefly, muscle mass is controlled by the relative rates of protein synthesis and catabolism. A major anabolic pathway is the canonical PI3K/AKT pathway which is activated by insulin, IGF-1 and other growth factors. Downstream of PI3K/AKT, the mammalian target of rapamycin (mTORC1) induces muscle hypertrophy (28,29). Activation of TORC1 stimulates mRNA translation and inhibition of apoptosis, causing an increase in cell size and number (30). Cellular degradation systems in skeletal muscle include the autophagy and ubiquitin-proteasome systems (29,31). Autophagy is a nonselective catabolic pathway through which damaged organelles and proteins are degraded. In the ubiquitin-proteasome system, proteins are targeted for degradation by the 26S proteasome, via protein ubiquitination. Muscle-specific ubiquitin protein ligases are considered the main enzymes responsible for targeting degradation of muscle structural and contractile proteins, and these include Tripartite Motif Containing 63 (TRIM63; also known as Muscle-Specific RING Finger Protein 1) and F-Box Protein 32 (FBXO32; also known as Atrogin-1 or Muscle Atrophy F-Box Protein) (29,31).
In the tumor-bearing host, reduced muscle protein synthesis and activation of catabolism occur, as a result of complex inflammatory, endocrine and nutrition-related effects. Cytokines and other pro-inflammatory molecules generated by host immune system-tumor interactions are thought to play a central role. Pro-inflammatory cytokines activate the hypothalamic-pituitary-adrenal axis, leading to production of catabolic stress hormones (adrenalin, cortisol, glucagon), generating resistance to insulin and growth factors in muscle, increased proteolysis and reduced protein anabolism (27,32). Transcriptional up-regulation of autophagy and ubiquitin-proteasome system is directly activated in muscle cells by a series of pro-inflammatory actors which originate in either the tumor, immune system or both. Prostaglandin (PG) E2 activates protein catabolism in skeletal muscle (33). Cytokine mediators of muscle catabolism include interleukin (IL)-6, IL-1, tumor necrosis factor-α, interferon γ leukaemia inhibitory factor and TNF ligand superfamily member 12 (TWEAK) (27,34). These factors signal through their respective cell surface receptors and activate the transcription of ubiquitin proteasome and autophagy genes. Nutritional deficits also play a role in the catabolic response of muscle cells. For example, amino acids, particularly the branched chain amino acid leucine, normally stimulate anabolism (and reduce catabolism) of protein in muscle (29,35,36). Low plasma concentrations of amino acids are permissive for activation of the ubiquitin system, autophagy and apoptosis (28,36).
Low muscle radiation attenuation reflects excess accumulation of lipid
Further studies are needed to identify the specific physiological mechanisms that result in reduced muscle radiation attenuation, as this has been only recently described and the biological findings are sparse. Low radiation attenuation is associated with accumulation of lipid (37) and the term myosteatosis is often used to describe it (25,26). In patients with cancer cachexia, rectus abdominis muscle evaluated by transmission electron microscopy showed increased number and size of intra-myocellular lipid droplets compared with non-weight losing controls (38). Preliminary results from our research group also suggest high levels of intra-myocellular lipid in muscles of patients with cancer, revealed by staining rectus abdominis muscle biopsy with Oil Red O (Figure 3A,B), used in the morphological examination of neutral lipids (i.e., triglyceride). As well, large aggregates of adipocytes are evident in regions of the perivascular connective tissue (Figure 3C).
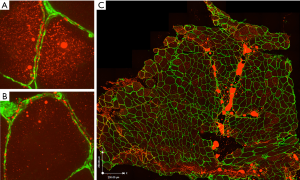
While mechanisms of excess fatty infiltration are unclear, Stretch et al. (39) performed transcriptomic analysis in rectus abdominis muscle biopsies in patients with cancer who had low versus high muscle radiation attenuation values. Differentially expressed genes associated with low muscle attenuation were involved in cell death and survival, cellular function and maintenance, and cell morphology. Oxidative phosphorylation was the most strongly affected canonical pathway. Eighteen differentially expressed genes associated with this pathway (encoding proteins in complex I, II, IV and V of the electron transport chain), had lower expression in muscles from patients with low muscle radiation attenuation. Decreased lipid oxidation would be expected to contribute to lipid accumulation seen in myosteatosis.
Clinically, low radiation attenuation in muscle of patients with cancer has been shown several times to be correlated with the presence of systemic inflammation as assessed by CRP or Glasgow Prognostic Score (25,40,41). Preliminary evidence suggests that myosteatosis is preventable or reversible by the provision dietary ω-3 fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], both clinically (42) and experimentally (43), and this may be due to the well-characterized anti-inflammatory action of these fatty acids.
Tumor- and treatment-driven muscle catabolism
Tumor-generated catabolism: how do we counteract it?
Malignant tumors contribute prominently to muscle wasting by merit of their direct capture of energy fuels and amino acids and their consequent ability to deprive other tissues of these substrates (44). Tumor cells also generate numerous catabolic factors that directly activate proteolysis in skeletal muscle. These include but are not limited to eicosanoids, cytokines (see above), members of the transforming growth factor (TGF)-β superfamily [activins, myostatin, TGFβ 1 and 2, growth and differentiation factor (GDF)-11 and GDF-15] (27,34). Muscle response to these effectors is via cell surface receptors, which are linked to transcriptional activation of ubiquitin, proteasome and autophagy genes.
The degree to which tumor-generated muscle catabolism is modifiable is not clear. Muscle gain does occur in patients with advanced cancer, and this seems to occur in the context of disease response to anticancer treatment (45). There is clinical evidence that muscle anabolism can be activated under appropriate conditions. Patients with locally advanced or metastatic disease demonstrate activation of muscle protein synthesis after intake of high-quality proteins (46,47). We do not yet possess a means of eliminating the chorus of catabolic effectors noted above, although several of those molecules are the targets of investigational new therapies. Several drugs have been shown to induce increases in muscle mass, even in patients with some of the most catabolic diseases, including advanced lung cancer and cholangiocarcinoma. These include anamorelin® (a growth hormone secretagogue receptor type 1 (ghrelin receptor) agonist) (48), as well as selumetinib® which targets mitogen-activated protein kinase kinases (49). Optimum conditions for exploitation of this anabolic potential are currently under study, with the overall aim of net improvement in the muscle mass, functionality, performance status and treatment tolerance by the patient (50).
Dietary ω-3 polyunsaturated fatty acids have been proposed as therapeutic agents for treatment and prevention of muscle loss (51,52). The main studied effect of ω-3 fatty acids is to down—regulate the synthesis of catabolic pro-inflammatory eicosanoids (PGE2), cytokines (TNF-α, IL-6, IL-1β), and their downstream effectors such as NF-κB, that induce muscle proteolysis (53-55). Clinically ω-3 fatty acids increase the rate of muscle protein synthesis in older adults (56) and in a recent meta-analysis (51) high-protein ω-3 polyunsaturated fatty acid-enriched oral nutritional supplements supported weight gain (+1.89 kg, 95% CI, 0.51–3.27, P=0.02) and provided attenuation of lean body mass loss in patients with cancer on chemotherapy, versus an isocaloric control.
When cancer is unresponsive to treatment and showing rapid growth, loss of muscle mass occurs at high rates (45). In disseminated metastatic disease, the mass and high metabolic activity of the tumor relentlessly drive muscle and fat loss and this accelerates during the last 3 months of life (45,57). There is a point in the trajectory of incurable cancer where this intense catabolism is unstoppable. The term “refractory cachexia” (1) was coined to describe a rapidly progressive cancer unresponsive to anticancer therapy, and a corresponding state of highly active catabolism, to a point that renders active management of muscle loss impossible.
Chemotherapy-induced muscle catabolism: a treatment side effect that can be mitigated?
The radiological approach to the assessment of muscle wasting has provided new quantitative information regarding the involvement of cancer treatments on muscle wasting. Cancer treatments often elicit losses of weight and of muscle, and these effects can be substantial. Weight loss of 4–12 kg easily occur during a standard course of neoadjuvant chemotherapy or chemo-radiotherapy (58-60). Diagnostic imaging reveals that these losses are often composed mostly of muscle (22,58,60).
To at least some extent cancer treatments induce loss of muscle mass via gastrointestinal side effects such as anorexia, oral mucositis, dry mouth, early satiety, malabsorption, diarrhea, nausea and vomiting. During cancer treatment patients experience additional problems that contribute to poor food intake, such as pain, anxiety, depression, altered sleep, fatigue and endocrine disorders. For many of these side effects there are therapeutic options. The management of these issues should be prioritized, as they may be readily reversed by appropriate treatments (e.g., pain, nausea, reduced bowel motility, mood disorders). A team approach, involving oncologists, palliative care physicians, dietitians, patients and families is required to optimize this approach. Pain and symptom management is crucial to maintain or improve food intake during treatment. Updated evidence-based clinical practice guidelines for nutrition in clinical oncology are available (52). Nutrition counseling by an accredited health care professional working within the supportive care team provides patients with a thorough understanding of their nutritional needs and of the specific eating habits that they can undertake to meet those needs.
More recently, it is becoming clear there are direct effects of cytotoxic and targeted cancer therapies on muscle cells, including altered contractile properties, insulin resistance and atrophy. These side effects of cancer treatments are worrisome and for the moment are incompletely understood. Many cancers show aberrant activation in pathways upstream of the mammalian target of rapamycin (mTORC1). For this reason, “targeted” cancer therapies, by design, are directed at the mTORC1 complex (e.g., sirolimus, everolimus and ridaforolimus) (30). Unfortunately muscle protein synthesis is activated by insulin and amino acids via the same (mTORC1-dependent) pathways that tumor cells rely on for proliferation (28,29), so the predicted effect of such agents is muscle atrophy. Furthermore, several cytotoxic agents (e.g., oxaliplatin, cisplatin, anthracyclines, 5-fluorouracil, irinotecan) appear to be taken up by muscle cells and induce proteolytic and apoptotic signaling, mitochondrial dysfunction, oxidative damage, cellular energy depletion and apoptotic or necrotic cell death (summarized in 34). In mice on gemcitabine + cisplatin therapy, skeletal muscles showed induction of TGFα family ligands myostatin and activin A, pro-inflammatory cytokines TNF-α, IL-6 and IL-1β, as well as the expression of ubiquitin ligases TRIM63 and FBXO32 and proteasome activity (61). These direct effects of cancer therapeutics on muscle suggests that debilitating muscle atrophy is a significant (and under-appreciated) adverse effect of cancer treatment.
Post scriptum: limitations and opportunities
Cancer-associated loss of skeletal muscle sits at the center of our current conception of cancer-associated cachexia (1). With the advent of secondary analysis of standard oncologic images, muscle mass and muscle loss are now precisely quantifiable, and this approach is being used to generate detailed assessments in different cancer types and treatment plans. There have been extensive studies in animal models, but one of the main limitations has been the sparsity of our knowledge of the human biology of cancer-associated muscle wasting. We lack understanding of molecular mechanisms underlying the heterogeneity of cachexia in individual patients with the same pathologic type of cancer. A positive development is that tissue-level investigations are accessible through collaboration with surgeons to obtain intraoperative muscle biopsy during cancer surgery [e.g., (38,39)]. These types of approaches will provide much-needed mechanistic insights.
It is becoming increasingly clear that an important part of the muscle loss experienced by cancer patients is iatrogenic. Paradoxically, two of the major long-standing treatments for cancer anorexia and cachexia, corticosteroids and progestational agents, have among their major side effects, atrophy of skeletal muscle (62). A wide variety of systemic antineoplastic agents generate muscle loss directly by expressing direct catabolic actions on muscle cells, as well as secondarily via their systemic (gastrointestinal) side effects that impair food intake during treatment. Muscle wasting deserves consideration as a potential adverse effect in the use of current cancer therapies and the development of new ones. Where muscle wasting is most profound, consideration might also be given to adding preventative measures to limit the toll of this side effect.
While we know that some patients with cancer have sarcopenia at diagnosis [e.g., (10)], the year of death is the period in which the most striking catabolic losses of muscle ensue (45,57). This is illustrated by CT images (Figures 1,2); images from the end of life show that some patients have become frankly emaciated. In our setting, about one in five patients with advanced cancer reach this body habitus. In current paradigms of care, a patient entering the year of death is likely to be an outpatient at a cancer center or hospital, in the charge of an oncologist, receiving chemotherapy and have access to referral for supportive care in various forms within the institution. Towards the end of the year of death, treatment may or may not still be ongoing (63), and referral for palliative care is likely to have occurred. Muscle wasting and cachexia are not generally a primary focus of oncologists, and the opportunity for early identification and intervention can easily be lost. Referral to palliative care still occurs late in the disease trajectory for many patients, at which time cachexia and its associated muscle wasting may have reached the refractory stage. There are current calls for the integration of oncology and palliative care (64): the year of death trajectory of cancer cachexia is but one example of the need for integration, in this case of diagnostic imaging, human and experimental biology, supportive and palliative care, clinical nutrition, clinical pharmacology and oncology.
Acknowledgements
VE Baracos is supported by the Alberta Cancer Foundation and the Canadian Institutes of Health Research. VC Mazurak is supported by the Canadian Institutes of Health Research. AS Bhullar holds a scholarship from Alberta Innovates Technology Futures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115-22. [Crossref] [PubMed]
- MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care 2011;5:342-9. [Crossref] [PubMed]
- Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 2009;3:269-75. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Sjøblom B, Grønberg BH, Wentzel-Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr 2016;35:1386-93. [Crossref] [PubMed]
- Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012;17:1240-5. [Crossref] [PubMed]
- Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage 2012;44:181-91. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Caan BJ, Cespedes Feliciano EM, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018;4:798-804. [Crossref] [PubMed]
- Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131-40. [Crossref] [PubMed]
- Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. J Clin Oncol 2014;32:2611-6. [Crossref] [PubMed]
- Pichard C, Baracos V, Attaix D. Would you buy a new tool to improve your practice? Curr Opin Clin Nutr Metab Care 2011;14:221-2. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920-6. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007;13:3264-8. [Crossref] [PubMed]
- Vrieling A, Kampman E, Knijnenburg NC, et al. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus 2018;4:420-34. [Crossref] [PubMed]
- Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333-8. [Crossref] [PubMed]
- Recio-Boiles A, Galeas JN, Goldwasser B, et al. Enhancing evaluation of sarcopenia in patients with non-small cell lung cancer (NSCLC) by assessing skeletal muscle index (SMI) at the first lumbar (L1) level on routine chest computed tomography (CT). Support Care Cancer 2018;26:2353-9. [Crossref] [PubMed]
- Derstine BA, Holcombe SA, Goulson RL, et al. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J Nutr Health Aging 2017;21:180-5. [PubMed]
- Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol 2018;29:ii1-9. [Crossref] [PubMed]
- Blauwhoff-Buskermolen S, Versteeg KS. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol 2016;34:1339-44. [Crossref] [PubMed]
- Antoun S, Lanoy E, Iacovelli R, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;119:3377-84. [Crossref] [PubMed]
- Chu MP, Lieffers J, Ghosh S, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298-304. [Crossref] [PubMed]
- Malietzis G, Johns N, Al-Hassi HO, et al. Low Muscularity and Myosteatosis Is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg 2016;263:320-5. [Crossref] [PubMed]
- Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489-97. [Crossref] [PubMed]
- Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. [Crossref] [PubMed]
- Adegoke OAJ, Abdullahi A, Tavajohi-Fini P. mTORC1 and the regulation of skeletal muscle anabolism and mass. Appl Physiol Nutr Metab 2012;37:395-406. [Crossref] [PubMed]
- Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014;49:59-68. [Crossref] [PubMed]
- Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med 2011;89:221-8. [Crossref] [PubMed]
- Sandri M. Protein breakdown in cancer cachexia. Semin Cell Dev Biol 2016;54:11-19. [Crossref] [PubMed]
- Braun TP, Zhu X, Szumowski M, et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic–pituitary–adrenal axis. J Exp Med 2011;208:2449-63. [Crossref] [PubMed]
- Strelkov AB, Fields AL, Baracos VE. Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol 1989;257:C261-9. [Crossref] [PubMed]
- Schiessel DL, Baracos VE. Barriers to cancer nutrition therapy: excess catabolism of muscle and adipose tissues induced by tumour products and chemotherapy. Proc Nutr Soc 2018;77:394-402. [Crossref] [PubMed]
- Barazzoni R, Short KR, Asmann Y, et al. Insulin fails to enhance mTOR phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. Am J Physiol Endocrinol Metab 2012;303:E1117-25. [Crossref] [PubMed]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9-22. [Crossref] [PubMed]
- Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104-10. [Crossref] [PubMed]
- Stephens NA, Skipworth RJ, Macdonald AJ, et al. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle 2011;2:111-7. [Crossref] [PubMed]
- Stretch C, Aubin JM, Mickiewicz B, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One 2018;13:e0196235. [Crossref] [PubMed]
- Okugawa Y, Toiyama Y, Yamamoto A, et al. Close Relationship Between Immunological/Inflammatory Markers and Myopenia and Myosteatosis in Patients With Colorectal Cancer: A Propensity Score Matching Analysis. JPEN J Parenter Enteral Nutr 2018. [Epub ahead of print]. [PubMed]
- McSorley ST, Black DH, Horgan PG, et al. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr 2018;37:1279-85. [Crossref] [PubMed]
- Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab 2014;39:654-62. [Crossref] [PubMed]
- Almasud AA, Giles KH, Miklavcic JJ, et al. Fish oil mitigates myosteatosis and improves chemotherapy efficacy in a preclinical model of colon cancer. PLoS One 2017;12:e0183576. [Crossref] [PubMed]
- Friesen DE, Baracos VE, Tuszynski JA. Modeling the energetic cost of cancer as a result of altered energy metabolism: implications for cachexia. Theor Biol Med Model 2015;12:17. [Crossref] [PubMed]
- Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012-9. [Crossref] [PubMed]
- van Dijk DP, van de Poll MC, Moses AG, et al. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J. Cachexia. Sarcopenia Muscle 2015;6:212-21. [Crossref] [PubMed]
- Engelen MPKJ, Safar AM, Bartter T, et al. High anabolic Potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann Oncol 2015;26:1960-6. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Prado CMM, Bekaii-Saab T, Doyle LA, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 2012;106:1583-6. [Crossref] [PubMed]
- Baracos VE. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol 2015;16:13-4. [Crossref] [PubMed]
- de van der Schueren MA, Laviano A, Blanchard H, et al. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol 2018;29:1141-53. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos VE, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition 2015;31:549-55. [Crossref] [PubMed]
- Di Giorgio FG, Girolamo D, Situlin R, et al. Omega-3 fatty acids and protein metabolism : enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care 2014;17:145-50. [Crossref] [PubMed]
- Schiessel DL, Yamazaki RK, Kryczyk M, et al. Does Oil Rich in Alpha-Linolenic Fatty Acid Cause the Same Immune Modulation as Fish Oil in Walker 256 Tumor-Bearing Rats ? Nutr Cancer 2016;68:1369-80. [Crossref] [PubMed]
- Smith GI, Atherton P, Reed DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93:402-12. [Crossref] [PubMed]
- Lieffers JR, Mourtzakis M, Hall KD, et al. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 2009;89:1173-9. [Crossref] [PubMed]
- Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 2012;31:74-7. [Crossref] [PubMed]
- Kubrak C, Olson K, Jha N, et al. Clinical determinants of weight loss in patients receiving radiation and chemoirradiation for head and neck cancer: A prospective longitudinal view. Head Neck 2013;35:695-703. [Crossref] [PubMed]
- Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 2007;29:893-900. [Crossref] [PubMed]
- Chen MC, Hsu W, Hwang P, et al. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016;7:51608-618. [PubMed]
- Baracos VE, Watanabe S, Fearon K. Aetiology, classification, assessment & treatment of anorexia-cachexia. In: Cherny N, Fallon M, Kaasa S, et al. editors. Oxford Textbook of Palliative Medicine, 5th edition, N Cherney Ed., Oxford University Press 2015:702-12.
- Grendarova P, Sinnarajah A, Trotter T, et al. Variations in intensity of end-of-life cancer therapy by cancer type at a Canadian tertiary cancer centre between 2003 and 2010. Support Care Cancer 2015;23:3059-67. [Crossref] [PubMed]
- Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 2018;19:e588-653. [Crossref] [PubMed]


