
External beam radiation therapy (EBRT) for asymptomatic bone metastases in patients with solid tumors reduces the risk of skeletal-related events (SREs)
Introduction
Bone metastases are common in cancer patients, affecting about 65–75% of patients with either breast cancer or prostate cancer and 30–40% of patients with lung cancer (1). External beam radiation therapy (EBRT) is often recommended when symptomatic bone metastases are identified (2). The most serious complications of bone metastases include pathological bone fractures and compression of the spinal cord or nerve roots due to vertebral collapse. These skeletal-related events (SREs) and the pain associated with bone metastases have devastating consequences for patient mobility, social functioning, and quality of life (3-5). SREs are also associated with a decrease in patient survival (2,6,7) and constitute a substantial financial burden. A single SRE was estimated, in 2004, to add $12,000 to the cost of treatment for a patient with lung cancer (8), a figure which had risen to $28,000 when the same estimate was made 5 years later (9).
The role of EBRT in controlling the complications of bone metastases is amply documented (10-13), but the optimal approach to palliative radiotherapy (RT) in this setting remains uncertain. Research performed in the 1970’s failed to demonstrate a clear relationship between pain relief and either radiation dose or the radiosensitivity of the primary tumor (14,15) resulting in wide variations in the fractionation schedules adopted by clinicians (16). Later studies focusing on the efficacy of single-fraction vs multiple-fraction dosing found that both schedules reduced the risk of skeletal complications to an identical degree (17-21). The accumulating evidence on the dosing of radiation therapy did not, however, resolve the question of when treatment ought to be initiated. In the absence of data, clinicians have necessarily relied upon personal judgements and habits acquired during training. The result is often that EBRT is not considered until bone metastases have given rise to pain, a strategy which entails withholding treatment from the 60% of bone metastases that are asymptomatic at the time of discovery (22,23). This figure is likely to rise as imaging and surveillance methods improve (24-26).
With these observations as background, we undertook a review of EBRT and bone metastases in a single cancer center to address two clinically important questions: (I) How frequently is EBRT being administered to patients with asymptomatic bone metastases? and (II) Is the use of EBRT in patients with asymptomatic bone metastases associated with a delay in the first occurrence of moderate-to-severe pain and SREs?
Methods
Study design and patients
A retrospective manual chart review was conducted for patients who received a diagnostic code indicating metastatic bone disease. All were treated at a single cancer center from 2007 to 2017. Patients with bone metastases were eligible for the study if they reported no pain on a 10-point scale and had no history of pathological fracture or spinal cord compression. Covariates included in the analysis were age, sex, cancer type, Eastern Cooperative Oncology Group (ECOG) status, number of bone metastases (≤3 vs. >3), presence of brain or visceral metastases, prior systemic therapy (chemotherapy or immunotherapy), location of metastases (axial, appendicular or axial and appendicular), prior non-EBRT bone-targeted therapy (bisphosphonate, denosumab or Ra-223) and type of metastasis (blastic, lytic or mixed). More exact location of metastases was not feasible due to the limitations of the clinical record and the variability of potential sites.
Patients with newly diagnosed asymptomatic bone metastases who received EBRT formed the treated group and were compared to patients who did not receive EBRT. The time elapsed from the diagnosis of asymptomatic bone metastases to either the onset of moderate-to-severe pain or the first SRE was recorded for both treated and untreated groups. Pain was considered moderate-to-severe if it required palliation with EBRT or was rated at least 5 out of 10 by the patient. The date assigned to an SRE was the date that a pathological fracture or spinal cord compression was first documented. EBRT dosing protocols were variable and were not included in the analysis.
Statistical methods
Wilcoxon rank sum tests, chi-square tests and Fisher’s exact tests were used to compare the demographic and tumor characteristics of treated and untreated patients. The Kaplan-Meier method and log-rank test were used to characterize the treated and untreated groups by time to SRE and overall survival (OS), the two measures of outcome. The contribution of selected covariates to the outcome measures for the combined groups was analyzed with a Cox proportional hazards regression model. All statistical tests were two-sided and adopted a 5% type I error.
The study was approved by Institutional Review Board of Fox Chase Cancer Center (No. 17-9034).
Results
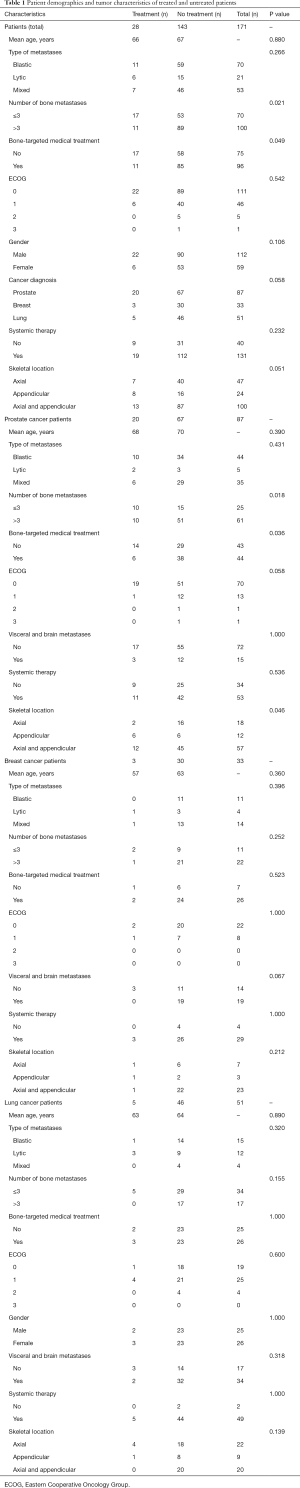
Asymptomatic bone metastases were identified in 171 patients: 87 with prostate cancer, 51 with lung cancer, and 33 with breast cancer. Twenty-eight of the 171 patients (16%) were prescribed EBRT upon diagnosis of asymptomatic bone metastases. Table 1 contains the demographic, clinical, and pathological data characterizing treated and untreated patients. The mean age of patients receiving EBRT for asymptomatic bone metastases was 66, nearly identical to the mean age of 67 for the untreated group. Patients in both the treated and untreated groups usually received systemic therapy, i.e., chemotherapy or immunotherapy. Use of systemic therapy, metastasis type—blastic, lytic or mixed—and the presence of visceral or brain metastases did not distinguish the treated and untreated patients for any of the subgroups; these variables were excluded from the multivariate analysis.
Full table
Univariate analysis demonstrated that patients with prostate cancer were more likely to receive EBRT for asymptomatic bone metastases if they had 3 or fewer metastases (P=0.021) or if they had previously received bone-targeted medical therapy (bisphosphonate, denosumab or Ra-223; P=0.049). The findings were similar when all cancer types were combined. For breast and lung cancer patients, however, neither the number of metastases nor prior bone-targeted medical therapy predicted the likelihood of receiving EBRT.
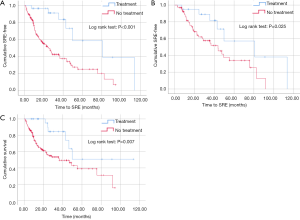
For the combined cancer groups, median time from the diagnosis of asymptomatic bone metastases to pain or an SRE was 25 months for the untreated patients and 81 months for the patients treated with EBRT (Figure 1A; P<0.001). When patients were divided by cancer type, EBRT was found to delay the occurrence of pain or an SRE for patients with prostate cancer from 44 months to 81 months (Figure 1B; P=0.025). Median time to an SRE could not be computed for lung cancer, although EBRT was associated with a delay in skeletal complications that was again significant (P=0.029). In contrast, EBRT did not have a statistically significant effect on time to moderate-or-severe pain or an SRE for patients with breast cancer.
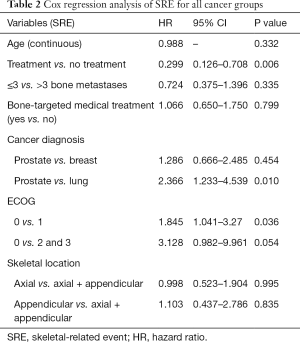
In a multivariate analysis, EBRT was again shown to reduce the risk of developing pain or an SRE when all cancer types were combined (Table 2; P=0.006). Among the covariates, specific cancer type and ECOG status, but not number or location of bone metastases, significantly influenced time to pain or an SRE.
Full table
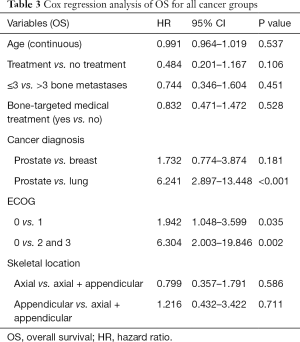
Differences in OS related to treatment and cancer diagnosis were also examined. The median OS for the prostate, breast, and lung groups was 71 months, 57 months and 17 months, respectively. Multivariate analysis demonstrated that the difference in OS between prostate patients and lung patients was significant (P<0.001). The effect of treatment on OS was examined using the Kaplan-Meier method. No treatment effect on OS was shown when the prostate, breast and lung cancer groups were analyzed separately. When all cancer types were combined, survival analysis demonstrated that OS was greater for patients with asymptomatic bone metastases who received EBRT (Figure 1C; P=0.007). However, the association of EBRT with improved OS was not reproduced in a multivariate analysis using a proportional hazards regression model (Table 3).Thus, OS was associated with cancer type and favored the prostate patients but did not differ for the treated and untreated patients.
Full table
Discussion
We conducted a retrospective chart review of 171 prostate, lung and breast cancer patients with asymptomatic bone metastases. Two principal findings emerged: (I) EBRT was used in a small minority of these patients—only 16%; and (II) EBRT, for all cancer types combined, was associated with a delay in the onset of skeletal complications from a median of 25 months to a median of 81 months. A separate analysis performed for each cancer type demonstrated a statistically significant benefit for EBRT in prostate and lung cancer patients but not in patients with breast cancer. This benefit cannot be explained by differences in OS, which did not differ for the treated and untreated patients. The findings cast doubt on the common practice of initiating EBRT only after bone metastases have given rise to symptoms. Indeed, this practice has already come into question in recent years in response to at least two critical developments.
Progress in bone-targeted medical therapy—bisphosphonates, bone-seeking radiopharmaceuticals, and monoclonal antibodies—has had a major impact on cancer treatments and outcomes. The bisphosphonates (pamidronate and zoledronic acid, among others) are highly potent, cost-effective nitrogen-containing agents that inhibit osteoclast-mediated bone resorption, reducing the incidence of bone metastases and preventing further complications from metastases once they form (27-30). A 2013 study found that 88% of Canadian breast cancer patients with known bone metastases had received bone-targeted medical therapy (23). One-half of these patients had suffered an SRE before treatment was begun. For such patients, nationwide protocols stipulating that treatment should pre-date the first SRE had not been followed. The risk of an additional SRE in that group was 83%. For patients who received treatment in the recommended fashion, i.e., before developing any skeletal complications due to bone metastases—the risk of an SRE was only 31%.
Similar results were reported in a study comparing the efficacy of zoledronic acid and pamidronate (5). When zoledronic acid was initiated for asymptomatic bone metastases, this agent delayed the onset of the patients’ first SRE by 522 days. For patients who received identical treatment only after their bone lesions had become painful, the time to the first SRE was reduced to 10 days.
Radiopharmaceuticals and monoclonal antibodies have also accelerated the trend toward treatment of bone metastases before they give rise to complications. A prospective study of breast and lung cancer patients examining the efficacy of the radionuclide samarium-153 EDTMP reported “very good” pain relief in 18/21 treated patients and 0/18 controls. Treatment must begin, the authors concluded, “before the establishment of severe pain syndrome” (31). Wang and colleagues examined the combined use of the bisphosphonate zoledronic acid and the radiopharmaceutical Sr-89 to treat asymptomatic bone metastases in patients with non-small cell lung cancer (32). The median time to the first SRE was extended by almost 7 months for the treated patients.
Several studies have compared the efficacy and cost effectiveness of bisphosphonates and monoclonal antibodies when administered to patients with asymptomatic bone metastases. While monoclonal antibodies are marginally more effective than bisphosphonates, this advantage is offset by a substantial increase in cost (28,29,33,34). There are no data which allow one to contrast the cost-effectiveness, complication rate, and tolerability of EBRT with bisphosphonates or radiopharmaceuticals in the setting of asymptomatic bone metastases.
A second major influence on changing attitudes toward patients with asymptomatic bone metastases has been the recognition that more patients are living long enough for skeletal complications to develop (35). Many of the previously cited studies of single-fraction and multiple-fraction EBRT schedules for painful bone metastases noted that the prognosis of such patients was poor (12,36). Follow-up periods of a year or less were therefore judged to be appropriate. The patients’ limited prospects for survival also gave clinicians little incentive to treat bone metastases that were not causing pain. Even under the old perspective, however, there were exceptions to the conventional palliative approach. Surgery, followed by radiotherapy, was universally recommended for the asymptomatic patient with a large lytic lesion of the femur (a lesion which carries a high risk of pathological fracture) (37). Surgery was also indicated for patients with vertebral destruction in whom spinal cord compression seemed imminent (11). These exceptions to routine practice make clear that it is not possible to draw strict guidelines for the treatment of bone metastases based only on the presence of symptoms. Optimal use of EBRT, like other forms of palliative treatment, requires an assessment of the risk of future complications and an estimate of when such complications are likely to occur.
Several limitations of the design of this study have bearing on the reported results. Under-representation of the breast cancer patients—only 3 of whom received EBRT—made it unlikely that significant results would be achieved for this group. Thus, the failure to show a benefit of EBRT for breast cancer patients was not unexpected. As noted in Table 1, patients with ≤3 bone metastases were over-represented in the group receiving EBRT. This sampling error would tend to prolong survival in the group with fewer metastases. Multivariable analysis, however, demonstrated that the number of bone metastases had no significant effect on either OS or SREs (Tables 2,3). This unexpected finding may be explained by noting that many factors in addition to the number of bone metastases, including the location and extent of non-osseous metastases, determine the prognosis of patients with cancer.
An unavoidable feature of our study design was the variable length of patient follow-up associated with different cancer types. Lung cancer patients survived a mean of 17 months in this study. Prostate patients, who survived a mean of 71 months, were necessarily more exposed to late complications of bone metastases, and thus more likely to demonstrate the late benefits of EBRT. Patient selection in future studies should be guided by the knowledge that the benefits of EBRT will be more readily detected in patients with longer OS.
Detailed information regarding our patients’ bone metastases, including their size and precise location, could not be obtained from this retrospective chart review. Location of the metastases was therefore identified broadly as axial, appendicular or axial and appendicular, a variable which did not influence the risk of an SRE. Dosing schedules in this retrospective study were highly variable and included 3,000 cGy ×10, 2,700 cGy ×3, 1,600 cGy ×1, 800 cGy ×1, and 600 cGy ×5. The small number of patients receiving any one of the various dosing schedules meant statistical comparison of the groups could not be performed. These constraints on the data regarding metastasis location and dosing schedules are typical of a retrospective design employing relatively small, unevenly distributed patient groups and underscore the value of pursuing these findings with a large, prospective study.
Despite such limitations, our study offers clear evidence that the treatment of bone metastases with EBRT should not be guided exclusively by the presence of symptoms. This is not a surprising result in light of clinical experience. It is known that asymptomatic bone metastases may produce serious complications without clinical warning (19). Moreover, exclusive attention to the presence of symptoms ignores the fact that bone metastases differing in location, size or histology may differ in clinical course and response to EBRT. Classifying such lesions strictly by the presence or absence of pain will almost certainly lead to inadequate treatment strategies.
The conclusion that EBRT should be considered for asymptomatic bone metastases is consistent with a specialty-wide trend toward the management of cancer patients using individualized treatment protocols. Key variables to be included in future studies include the bulk and precise location of the bone metastases, the aggressiveness of the patient’s underlying tumor, the nature of patient comorbidities, and the relative efficacy of different RT dosing schedules. It would also be useful to identify which specific skeletal events (such as pain or pathological fracture) pose the greatest risk in a given clinical setting. A large, prospective, randomized clinical trial including all the aforementioned variables would help to stratify risk and to identify optimal candidates for early intervention. This study offers evidence that EBRT merits inclusion in such future trials and that EBRT may have a role in the treatment of bone metastases before they produce pain or other SREs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of Fox Chase Cancer Center (No. 17-9034).
References
- Coleman RE. Metastatic disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. [Crossref] [PubMed]
- Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007;110:1860-7. [Crossref] [PubMed]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997;69:1-18. [Crossref] [PubMed]
- Weinfurt KP, Castel LD, Li Y, et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care 2004;42:164-75. [Crossref] [PubMed]
- Costa L, Lipton A, Peyman H, et al. Treatment of bone metastases before the onset of pain. Int J Clin Oncol 2013;18:531-8. [Crossref] [PubMed]
- Hei YJ, Saad F, Coleman RE, et al. Fractures negatively affect survival in patients with bone metastases from breast cancer. Breast Cancer Res Treat 2005;94 suppl 1:S260.
- Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005;16:579-84. [Crossref] [PubMed]
- Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 2004;67:390-6. [Crossref] [PubMed]
- Langer C, Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: Demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer 2010;67:4-11. [Crossref] [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Falkmer U, Jarhult L, Wersall P, et al. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol 2003;42:620-33. [Crossref] [PubMed]
- Bates T. A review of local radiotherapy in the treatment of bone metastases and cord compression. Int J Radiat Oncol Biol Phys 1992;23:217-21. [Crossref] [PubMed]
- Arcangeli G, Giovinazzo F, Saracino B, et al. Radiation therapy in the management of symptomatic bone metastases: The effect of total dose and histology on pain relief and response duration. Int J Radiat Oncol Biol Phys 1998;42:1119-26. [Crossref] [PubMed]
- Garmatis CJ, Chu FC. The effectiveness of radiation therapy in the treatment of bone metastases from breast cancer. Radiology 1978;126:235-7. [Crossref] [PubMed]
- Jensen NH, Roesdahl K. Single-dose irradiation of bone metastases. Acta Radiol Ther Phys Biol 1976;15:337-9. [Crossref] [PubMed]
- Priestman TJ, Bullimore JA, Godden TP, et al. The Royal College of Radiologists' Fractionation Survey. Clin Oncol (R Coll Radiol) 1989;1:39-46. [Crossref] [PubMed]
- Gutiérrez Bayard L, Salas Buzón Mdel C, Angulo Paín E, et al. Radiation therapy for the management of painful bone metastases: Results from a randomized trial. Rep Pract Oncol Radiother 2014;19:405-11. [Crossref] [PubMed]
- Steenland E, Leer J, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. Erratum in: Radiother Oncol 1999;53:167. Leer, J [corrected to Leer, JW]; van Mierlo, T [corrected to van Mierlo, I]. [Crossref] [PubMed]
- Ratanatharathorn V, Powers WE, Moss WT, et al. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys 1999;44:1-18. [Crossref] [PubMed]
- Lam TC, Uno H, Krishnan M, et al. Adverse outcome after palliative radiation therapy for uncomplicated spine metastases: Role of spinal instability and single-fraction radiation therapy. Int J Radiat Oncol Biol Phys 2015;93:373-81. [Crossref] [PubMed]
- van der Linden YM, Steenland E, van Houwelingen HC, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol 2006;78:245-53. [Crossref] [PubMed]
- Koizumi M, Yoshimoto M, Kasumi F, et al. Post-operative breast cancer patients diagnosed with skeletal metastasis without bone pain had fewer skeletal-related events and deaths than those with bone pain. BMC Cancer 2010;10:423. [Crossref] [PubMed]
- Kuchuk I, Hutton B, Moretto P, et al. Incidence, consequences and treatment of bone metastases in breast cancer patients—Experience from a single cancer centre. J Bone Oncol 2013;2:137-44. [Crossref] [PubMed]
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [Crossref] [PubMed]
- Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J. Bone Joint Surg Br 2002;84:521-9. [Crossref] [PubMed]
- Sabino MA, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol 2005;3:15-24. [PubMed]
- Rove KO, Crawford ED. Evolution of treatment options for patients with CRPC and bone metastases: bone-targeted agents that go beyond palliation of symptoms to improve overall survival. Oncology (Williston Park) 2011;25:1362-70, 1375-81, 1387. [PubMed]
- Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 2008;19:420-32. [Crossref] [PubMed]
- Diel IJ. Bisphosphonates in the prevention of bone metastases: current evidence. Semin Oncol 2001;28:75-80. [Crossref] [PubMed]
- Kanis JA, Powles T, Paterson AH, et al. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone 1996;19:663-7. [Crossref] [PubMed]
- Gallicchio R, Giacomobono S, Nardelli A, et al. Palliative treatment of bone metastases with samarium-153 EDTMP at onset of pain. J Bone Miner Metab 2014;32:434-40. [Crossref] [PubMed]
- Wang Y, Tao H, Yu X, et al. Clinical significance of zoledronic acid and strontium-89 in patients with asymptomatic bone metastases from non-small-cell lung cancer. Clin Lung Cancer 2013;14:254-60. [Crossref] [PubMed]
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22. [Crossref] [PubMed]
- Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 2011;17:621-43. [Crossref] [PubMed]
- Gonzalez VJ, Howell K. 8 Gy single-fraction radiation for bone metastases: Do the data support a 1-size-fits-all approach? Pract Radiat Oncol 2017;7:16-8. [Crossref] [PubMed]
- Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life. Cancer 2010;116:2716-25. [PubMed]
- Heisterberg L, Johansen TS. Treatment of pathological fractures. Acta Orthop Scand 1979;50:787-90. [Crossref] [PubMed]


