
Lipid metabolism in cancer cachexia
Introduction
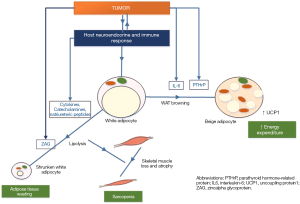
Cancer cachexia is a multifactorial syndrome, characterized by weight loss due to skeletal muscle and adipose tissue (AT) depletion, and is associated with high resting energy expenditure (REE), progressive functional impairment, poor quality of life and survixval (1,2). The extent of weight loss in cancer cachexia is unexplained by reduced food intake, and evidence suggests systemic inflammation to be central to its pathophysiology. Both tumor- and host- derived cytokines and immune peptides disrupt normal metabolism and are vital to the catabolic drive in cancer cachexia (3). Recently, an international consensus panel of experts considered muscle loss as an important inclusion criteria for cancer cachexia definition, while AT loss could be present or not (4). Indeed, muscle loss has long dominated cachexia research, and only recently the work conducted in the past decade and half has begun to unravel changes in AT morphology and function that are significant in the development and progression of cancer cachexia.
The view of AT as an inert energy storage depot has been replaced with the recognition that AT is a multi-compartment, heterogeneous and metabolically active organ with important endocrine and paracrine roles throughout the body (5). AT is diverse with respect to its anatomical location and cellular composition, and importantly, possesses a high degree of plasticity, adapting to physiological and pathological cues. For a long time, white AT (WAT) had been regarded as the only relevant AT in adults, and its loss in cancer cachexia attributed to an abundance of lipolysis factors consequent to the inflammatory tumor microenvironment. However, the demonstration of functional brown adipose tissue (BAT) in adults, and ability of white adipocytes to transform to “beige” adipocytes in response to tumor- and host-related factors has fundamentally changed this view. This review will discuss outline current knowledge related to the regulation of AT function and contributions of different types of AT in cancer cachexia.
AT types and function: white, brown and beige
The two main types of AT depots, WAT and BAT, have distinct functions of energy storage and heat generation, respectively. Based on the location and pathophysiological properties three categories of adipocytes are recognized: white, brown and beige.
WAT
In adult humans, WAT is the largest AT depot and the main energy reservoir in the body, and includes subcutaneous and visceral WAT. It is largely made up of white adipocytes specialized in the intake, storage and mobilization of lipids. It contains a unilocular lipid droplet that consumes most of the cell volume and a shell consisting of structural proteins and enzyme. Following food intake, excess free fatty acids (FAs) liberated from lipoproteins by lipoprotein lipase (LPL), are esterified to relative inert triacylglycerol (TAG) which is subsequently stored within lipid droplets. There is a constant flux of FAs entering and leaving these adipocytes, and TAG content depends on the net balance between lipogenesis (the biosynthesis, incorporation, and storage of TAG in the lipid droplet in the cytoplasm), and lipolysis (hydrolysis of TAG to FA and glycerol), which are tightly regulated predominantly by the sympathetic nervous system (SNS) and insulin (6,7). During periods of high energy demand such as fasting and exercise, TAG stores are mobilized to release FAs which are delivered to peripheral tissues for β-oxidation and ATP production (8). Lipolysis is achieved by three lipases, adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoglyceride lipase, which consecutively break down TAG to release FA and glycerol (Figure 1) (9).

The pivotal role of ATGL in TAG catabolism is evident from the severely reduced lipolysis and increased body-wide fat deposition in ATGL-deficient mice (10), and in humans with mutations of the ATGL gene (11,12). In contrast, HSL-deficient mice exhibit only a moderate decrease in stimulated lipolysis, and do not have increased fat deposition or obesity (10,13). An important ATGL regulatory mechanism is CGI-58 (comparative gene identification 58), which in basal conditions is sequestered with the lipid droplet-associated structural protein, perilipin-A to form an inactive complex, and is unable to bind to ATGL. During periods of high energy demands, lipolytic mediators, such as catecholamines which are the most important, and natriuretic peptides, bind to their respective adipocyte receptors, β-adrenoceptors (β-AR) and natriuretic peptide receptor A (NPRA), resulting in signaling that eventually activate protein kinase A (PKA) and G (PKG), respectively, which in turn promote the phosphorylation of HSL and perilipin-A. Phosphorylated perilipin-A frees CGI-58 to bind with ATGL and activate it. Phosphorylated HSL is able to translocate into the lipid droplet and together with ATGL accelerates the lipolytic process (Figure 1), Both ATGL and HSL are responsible for about 95% of the hydrolysis of TAG. ATGL represents the sole lipase activated by CGI-58 (12), and those with mutated CGI-58 have system wide fat deposition due to inability to activate ATGL (14,15). On the other hand, G0S2 (G0/S2 switch gene 2) has been identified as the major selective inhibitor of ATGL (16). Insulin is associated with potent antilipolytic effects mediated via activation of the enzyme phosphodiesterase 3 that decreases intracellular cyclic AMP levels, thereby inhibiting PKA activity, reducing HSL and perilipin phosphorylation (7).
BAT
Brown adipocytes, in contrast to white adipocytes contain multilocular lipid droplets, and is enriched with mitochondria, which gives it its brown color (17,18). The high expression of uncoupling protein 1 (UCP1) in the inner mitochondrial membrane, directly promotes thermogenesis by uncoupling oxidative phosphorylation from ATP synthesis to dissipate energy in the form of heat (19) UCP1 serves as a marker for brown fat activation. These thermogenic brown adipocytes are believed to be derived from a cell lineage different from white adipocytes, sharing a lineage with myocytes (20). The hallmark of BAT function is to expend energy (21), and for a long time functionally significant BAT was thought to be present in newborns, undergoing rapid involution with age (22). However, pockets of BAT depots are located in adults, near the aorta and within the supraclavicular region of the neck (23,24).
Browning of WAT
An additional type of adipocyte, “brite” (brown-in-white) or “beige” adipocyte has recently been identified. Beige adipocytes appear within WAT depots in response to sustained exposure to cold or β3-adrenergic stimuli, as well as to tumor related factors (25,26). These adipocytes are thought to share cell lineage with white adipocytes, but express UCP1 and function like brown adipocytes in expending energy (23). The progressive switch or remodeling of white-to-beige phenotype is referred to as WAT browning. At the molecular level, transcriptional events specifying differentiation into brown adipocytes are regulated by Peroxisome proliferator-activated receptor gamma (PPARγ), PPARγ coactivator 1-alpha (PGC-1α) and the transcription factor PR domain containing 16 (PDRM16), which physically interacts with CCAAT/enhancer-binding proteins C/EBPβ (27,28). The expansion of beige AT in cancer patients is being recognized as an important driver of cachexia (29), and discussed later in this review.
AT loss in cancer cachexia
Depletion of AT has an emerging role in the development and progression of cancer cachexia. In experimental models of cachexia, the losses in AT appear before the reductions in skeletal muscle mass and food intake (30,31). Similarly, a longitudinal body composition (dual-energy X-ray absorptiometry) study of predominantly gastrointestinal cancer patients (n=311) (32), showed the loss in body fat to occur more rapidly and earlier than lean tissue, and occurred preferentially from the trunk, followed by leg and arm. Another study in advanced cancer patients with various solid tumors demonstrated accelerated AT loss to begin 7 months prior to death, with an average loss of 29% at 2 months before death (33). In this study, the AT losses occurred in tandem with the losses in plasma phospholipid FAs and were predictive of survival. Studies also suggest that the shrinkage in size of AT depots is from depletion of lipid reserves that results in significantly smaller adipocyte cell size, and not due to the decrease in the number of cells (cell death) (34-37). The mechanism of AT loss in cancer cachexia has been attributed to increases in lipolytic activity and lipid utilization (37), while other mechanisms such as impaired adipogenesis and lipogenesis may also be contributory
Increased lipolytic activity and evidence for AT-muscle cross talk
Accumulating evidence suggests enhanced lipolysis to be central to AT loss in cancer cachexia. High lipolytic rate, as measured by fasting plasma glycerol or fatty acids in relation to body fat, has been observed in murine tumor cachexia models and cachectic cancer patients (25,26,38-42). Enhanced lipolysis is also suggested by the elevated expression of several lipolytic enzymes and mediators. Upregulation of HSL mRNA and protein expression in WAT has been shown in cachectic cancer patients (25,26,40). In one study, Thompson et al. demonstrated a twofold increase in HSL mRNA in subcutaneous WAT that significantly correlated with the twofold elevation in serum TAG and free FA levels (40). In another study, weight loss in cancer patients was accompanied by increased HSL expression and 2–3 folds increase in lipolytic effects of catecholamines and natriuretic peptides in adipocytes from subcutaneous WAT, which was completely blocked by an HSL-specific inhibitor (25). Das et al. demonstrated increased ATGL activity to correlate with tumor growth and WAT loss, in two murine cachexia models, and increased HSL and ATGL in visceral WAT of cancer patients to correlate negatively with BMI (38). In functional studies, ATGL-knockout mice were shown to be protected from WAT lipolysis (38). In addition to the prevention of WAT loss, an interesting observation was the preservation of skeletal muscle mass and prevention of muscle catabolic activities, suggesting an important cross-talk between the AT and muscle in cancer cachexia. HSL-knockout mice also exhibited similar resistance to fat and muscle loss, but it was to a lesser extent (38).
Several tumor and/or host derived factors have been implicated in AT depletion during progressive cachexia (3,38,43-46). These factors include inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin (IL)-1, IL-6 as well as the lipid mobilizing factor, zinc-α2-glycoprotein (ZAG). Furthermore, catecholamines (in mice and humans) and natriuretic peptides (humans) have higher ability to induce lipolysis in cancer cachexia. In the study by Das et al., elevation in pro-inflammatory cytokines and ZAG levels occurred during the early and intermediate phase of cancer cachexia when food intake was normal (38). Higher expression of lipolytic receptors, β1-AR has been demonstrated in WAT of cachectic cancer patients with a positive correlation between the expression of β1-AR protein and HSL (26). The expression of ZAG has been shown to be enhanced and to stimulate lipolysis in rodent adipocytes (47,48). In cachectic patients, enhanced ZAG expression in AT of cachectic cancer patients correlated with weight loss and was inversely correlated with BMI (35). The excess FAs from increased lipolytic activity are subsequently oxidized by mitochondria, and an upregulation in genes regulating mitochondrial lipid oxidation was observed in animal models and in patients with cachexia (34,37). Increased expression of adipocyte-specific gene cell death-inducing DNA fragmentation factor-α-like effector A (CIDEA), involved in the regulation of lipolysis and stimulation of FA oxidation, has been demonstrated in AT of cachectic patients, resulting in low basal lipolysis (49). Low CIDEA expression and high HSL and ATGL expression in cancer cachexia fat cells make these cells prone to increased activation of lipolysis following hormonal stimulation (9).
WAT browning and adaptive thermogenesis in cancer cachexia
The association between brown fat in cancer patients and increased REE in cancer cachexia has been suspected for a long time (3,50). Cachectic C26 colon carcinoma-bearing mice had higher number of BAT positive sites per 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG PET), as compared to non-tumor bearing mice, suggesting a role in cachexia (51). In humans, 18F-FDG PET studies revealed increased BAT in cancer patients as compared to healthy age-matched controls in one study (50), while another found a majority of adults had presence of BAT, with no difference between cancer patients and controls (52). Well-designed studies are therefore needed to understand the role of BAT in human cancer cachexia.
Recently, a major development in the cancer cachexia field has been in the recognition that white adipocytes undergo phenotypical changes converting to beige adipocytes (WAT browning) associated with increased thermogenic activity contributing to accelerated energy expenditure and propagation of cachexia in experimental tumor mouse models and cancer patients (17,18,29,53). The two recent pivotal independent studies in various cancer cachexia rodent models further provided the mechanistic link between the tumor and WAT browning in cancer cachexia. These studies confirm the generation of beige adipocytes in WAT, demonstrated by increased expression of UCP1 mRNA and protein, increased thermogenic activity, as an early event in the development and progression of AT and skeletal muscle wasting in cancer (17,18).
In the study by Spiegelman’s group, parathyroid hormone related peptide (PTHrP) was identified to have a significant role in adipocyte browning (17). The injection of anti-PTHrP antibody in LLC mice significantly decreased UCP1 expression in adipocytes, decreased energy expenditure and the severity of AT and muscle loss, but did not completely inhibit it (17), suggesting the involvement of other factors in WAT browning. The study also demonstrated increased expression of transcription regulators of brown adipogenesis such as Prdm16 and Pgc1α, and demonstrated UCP1 expression to be impaired in PRDM16 deficient LLC mice, with inhibition of AT loss (17), similar to another study demonstrating Prdm16 deficient mice to be resistant to browning (54). Furthermore, a significant proportion of lung and colon cancer patients (17 of 47 patients) had detectable PTHrP levels that was associated with lower lean body mass and higher REE (17).
In the second study, Petruzzelli and colleagues demonstrated higher UCP1 expression in WAT along with increased IL-6 expression in several cancer cachexia murine models (18). As compared to mice transplanted with tumor cells that did not express IL-6, mice transplanted with IL-6 expressing tumor cells developed substantial weight loss, and the use of an IL-6 blocking antibody or transplantation of melanoma cells into IL-6 receptor-deficient mice reduced but did not fully suppress cachexia (18). Blockade of β3adrenergic receptors using antagonists also significantly reduced the onset of cachexia in mice, which was associated with reduced expression levels of UCP1 in beige cells (18). In addition, the group found UCP1 expression in 7 of 8 samples of human AT from patients with colon cancer who have cachexia, and none from the 20 patients without cachexia (18).
Decreased adipogenesis and lipogenesis
In addition to lipolysis, AT loss may result from impaired adipogenesis and the ability to synthesize and store lipids in adipocytes. Reduced expression of adipogenic transcription factors have been demonstrated in various cancer cachexia models and associated with decreased adipocyte size (34,55) and higher TNF-α expression (34). Reduced adipocyte lipogenic enzyme expression such as fatty acid synthetase (FAS), has been demonstrated in animal tumor models and AT from cachectic cancer patients (30,56,57), with increase in liver lipogenesis and hypertriglyceridemia (58). Numerous animal studies also suggest reduced LPL activity in cancer (56,58-61). A study in colorectal cancer patients found decreased activity of LPL and FAS in visceral AT that was near the tumor as compared to distal AT (57). Reduced insulin sensitivity is commonly present in both human and animal models of cancer cachexia (25,46) and may contribute to AT loss via loss of lipogenic signaling and removal of inhibitory signaling to lipolytic pathways (Figure 1). More studies that determine the contribution of lipogenesis and adipogenesis at various stages of cancer cachexia are warranted.
Key mediators affecting AT in cancer cachexia
Multiple factors appear to be involved in the development and progression of cancer cachexia. The production of pro-inflammatory cytokines in response to the tumor, as well as tumor derived factors, affect AT directly or via their interaction with neuroendocrine pathways involved in energy regulation. Some of these factors that affect AT are discussed further.
Cytokines
TNF-α levels have been shown to be elevated in rodent models of cancer cachexia (31,38,62). In mice models, intraperitoneal injection of TNF-α expressing cancer cells promote weight loss, and the administration of neutralizing TNF-α antibodies averted the losses in WAT and lean mass (63,64). TNF-α stimulates lipolysis by several ways, including inactivation of insulin receptor signaling mediated through the p42/44mitogen-activated protein (MAP) kinase, thereby counteracting insulin’s anti-lipolytic activity (65). TNF-α also promotes lipolysis by decreasing the production of perilipin, which protects adipocytes from lipolysis (65). Currently, the role of TNF-α in human cancer cachexia is unclear. Serum TNF-α levels have been shown to vary considerably and most show no correlation with weight loss (3,25). Furthermore, trials of anti-TNFα antibodies have shown no benefit, suggesting that targeting TNFα alone is not sufficient to prevent cachexia (66,67).
IL-6 levels are increased in animal models of cancer cachexia (18,31,38,62,68), as well as in cachectic cancer patients (69-71), and the administration of anti-IL-6 monoclonal antibody shown to inhibit cachexia in tumor models and cancer patients (18,68,69). IL-6 decreases LPL activity as well as perilipin and PPARγ mRNA expression in 3T3-L1 adipocytes. In addition to enhancing lipolytic pathways, IL-6, as discussed previously, along with other factors is involved in WAT browning, and deregulation of lipid metabolism in BAT (53,72,73). The use of an IL6blocking antibody or transplantation of melanoma cells into IL6 receptor-deficient mice reduced browning and weight loss, but did not fully suppress cachexia (18).
Tumor factors
ZAG is a secreted soluble protein that is overexpressed in several malignant tumors (74), as well as in tissues such as WAT, BAT and heart (75,76). Tisdale and colleagues identified a lipid-mobilizing factor (LMF) identical to the protein ZAG identified from serum of mice bearing the cachexia-inducing MAC16 tumor as well as in urine and serum of cachectic cancer patients (77,78) ZAG expression has been associated with increased lipolysis in rodent adipocytes leading to AT and weight loss (47,48,79,80). The increase in weight loss has been shown to correlate with enhanced ZAG mRNA and protein in WAT and BAT of mice bearing the MAC16 tumor (48), and in AT of cachectic cancer patients (35). However, circulating ZAG levels have not correlated with weight or fat loss in two cancer studies (35,81).
ZAG induced lipolysis is thought to involve binding to β-AR in adipocytes (79), by stimulating adenylyl cyclase in a GTP-dependent process (47), and elevating HSL (35) and ATGL activity (38). While ZAG expression has been shown to occur in both wild type and ATGL knockout mice, only the wild type mice exhibited cachexia (38). ZAG expression appears to be regulated by glucocorticoids and the SNS. Glucocorticoids are proposed to increase lipolytic activity via increase in in ZAG expression (82,83). In cachectic cancer rodent models (84) and cancer patients (85), increased plasma cortisol levels have been associated with higher AT ZAG expression and elevated lipolysis. Both dexamethasone and a β3 agonist, BRL 37344, increased ZAG mRNA levels in 3T3-L1 adipocytes and stimulate lipolysis (83). Dexamethasone’s lipolytic activity is blocked by anti-ZAG monoclonal antibody suggesting dexamethasone mediated lipolysis is via induction of ZAG expression. The selective β3-AR receptor antagonist also completely attenuated the action of dexamethasone, suggesting that the ZAG produced moved into the medium and acted extracellularly though β3-AR (83).
PTHrP is overexpressed by many tumors and recently its presence in the circulation shown to be associated with greater degree of wasting in metastatic cancer patients (17). PTHRP and PTH signal through the PTH receptor, a G protein-coupled receptor (GPCR) that activates the cyclic-AMP-dependent PKA pathway, as do β-adrenergic receptors. Neutralization of PTHrP by a specific antibody attenuated wasting of both AT and skeletal muscle in tumor-bearing mice and mice lacking PTHR in AT are resistant to cachexia (17). Thus the PTH/PTHrP pathway may be and important target in cachexia therapeutics.
Transcriptional regulators of thermogenic adipocyte differentiation
The transcriptional regulation of the UCP1 gene is a key event in the process of adipocyte differentiation into thermogenic brown and beige adipocytes. At the molecular level, multiple transcriptions factors such as PPARy, PDRM16, PGC1α and C/EBPβ, regulate brown adipocyte-specific gene programs, downstream of β3-adrenergic receptor signaling pathways (27,28). In LLC mice, increased UCP1 expression in adipocytes occurred along with increased expression of PDRM16 and PGC1α (17), and UCP1 expression/browning is impaired in PRDM16 deficient mice with inhibition of AT loss (17,54). Therefore targeting pathways that inhibit PRDM16 and other regulators may lead to new therapeutic avenues.
Stress hormones and β-adrenergic stimulation and in AT wasting
Catecholamine activity, modulated by fasting, exercise and other hormones, is well known to activate PKA via β-AR (β1, β2 and β3) to induce lipolysis, and is also involved in BAT thermogenesis and WAT browning effects (7,86-90). Subcutaneous WAT from gastrointestinal cancer patients demonstrated β1-AR mRNA and protein expression to be significantly higher in cachectic patients than cancer controls and non-cancer weight losing controls (26). Expression of β1-AR protein positively correlated with lipolytic rate and HSL protein expression in this study, while other receptors such as β2-, β3 and α2-were unaltered in the cachexia group (26). However, in rodent models, β3-AR is recognized to be the predominant receptor on white and brown adipocytes (91), and ZAG promotes lipolysis by interacting with this receptor (79,83). The administration of β3-AR agonist induced marked increase in WAT lipolysis and BAT thermogenesis, while the agonist had no effect in β3-AR deficient mice did not (92,93). Increased β-adrenergic tone is implicated in WAT browning in cachexia as well as in obesity. Obesity research suggests the CNS, particularly the hypothalamus, as an important regulator of browning (94), and research to explore a similar role in cancer cachexia are needed.
Concluding remarks
AT’s role in cancer cachexia has evolved to a dynamic process, whereby a multitude of factors from the tumor and host, induce alterations in AT lipid metabolism particularly increased lipolysis and wasteful energy expenditure due to beige/brown adipocyte transformation (Figure 2). Additionally, an important crosstalk between AT and muscle loss has been suggested, as muscle loss appears to occur downstream to WAT lipolysis. While much progress has been made, additional research is clearly warranted to allow for a better understanding of the molecular mechanisms involved in AT alterations in the various stages of cancer cachexia, with the eventual goal to be able to develop interventions to improve this condition. Ongoing human trials for cancer cachexia appear promising, but none are directed toward AT’s role in cancer cachexia. Currently, two potential areas for therapeutics include inhibition of the key components and drivers of AT lipolytic activity and white-to-beige transformation. These therapies may have additional beneficial downstream effects on muscle mass, and could lead to improvements in patient reported outcomes such as physical functioning and hand grip strength.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cao DX, Wu GH, Zhang B, et al. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr 2010;29:72-7. [Crossref] [PubMed]
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90-9. [Crossref] [PubMed]
- Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology 2005;20:340-8. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc 2001;60:319-28. [Crossref] [PubMed]
- Nielsen TS, Jessen N, Jorgensen JO, et al. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol 2014;52:R199-222. [Crossref] [PubMed]
- Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471-82. [Crossref] [PubMed]
- Lass A, Zimmermann R, Oberer M, et al. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011;50:14-27. [Crossref] [PubMed]
- Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab 2014;25:255-62. [Crossref] [PubMed]
- Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006;312:734-7. [Crossref] [PubMed]
- Fischer J, Lefevre C, Morava E, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 2007;39:28-30. [Crossref] [PubMed]
- Schweiger M, Schreiber R, Haemmerle G, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006;281:40236-41. [Crossref] [PubMed]
- Okazaki H, Osuga J, Tamura Y, et al. Lipolysis in the absence of hormone-sensitive lipase: evidence for a common mechanism regulating distinct lipases. Diabetes 2002;51:3368-75. [Crossref] [PubMed]
- Lefèvre C, Jobard F, Caux F, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet 2001;69:1002-12. [Crossref] [PubMed]
- Schweiger M, Lass A, Zimmermann R, et al. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab 2009;297:E289-296. [Crossref] [PubMed]
- Yang X, Lu X, Lombes M, et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 2010;11:194-205. [Crossref] [PubMed]
- Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100-4. [Crossref] [PubMed]
- Petruzzelli M, Schweiger M, Schreiber R, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014;20:433-47. [Crossref] [PubMed]
- Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829-39. [Crossref] [PubMed]
- Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007;104:4401-6. [Crossref] [PubMed]
- Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 2013;154:2992-3000. [Crossref] [PubMed]
- Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol 1966;91:223-34. [Crossref] [PubMed]
- Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells 2014;6:33-42. [Crossref] [PubMed]
- Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518-25. [Crossref] [PubMed]
- Agustsson T, Ryden M, Hoffstedt J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res 2007;67:5531-7. [Crossref] [PubMed]
- Cao DX, Wu GH, Yang ZA, et al. Role of beta1-adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci 2010;101:1639-45. [Crossref] [PubMed]
- Ohno H, Shinoda K, Spiegelman BM, et al. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012;15:395-404. [Crossref] [PubMed]
- Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007;6:38-54. [Crossref] [PubMed]
- Kir S, Spiegelman BM. Cachexia & Brown Fat: A Burning Issue in Cancer. Trends Cancer 2016;2:461-3. [Crossref] [PubMed]
- Ishiko O, Nishimura S, Yasui T, et al. Metabolic and morphologic characteristics of adipose tissue associated with the growth of malignant tumors. Jpn J Cancer Res 1999;90:655-9. [Crossref] [PubMed]
- Byerley LO, Lee SH, Redmann S, et al. Evidence for a novel serum factor distinct from zinc alpha-2 glycoprotein that promotes body fat loss early in the development of cachexia. Nutr Cancer 2010;62:484-94. [Crossref] [PubMed]
- Fouladiun M, Korner U, Bosaeus I, et al. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care--correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005;103:2189-98. [Crossref] [PubMed]
- Murphy RA, Wilke MS, Perrine M, et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin Nutr 2010;29:482-7. [Crossref] [PubMed]
- Bing C, Russell S, Becket E, et al. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br J Cancer 2006;95:1028-37. [Crossref] [PubMed]
- Mracek T, Stephens NA, Gao D, et al. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer 2011;104:441-7. [Crossref] [PubMed]
- Rydén M, Agustsson T, Laurencikiene J, et al. Lipolysis--not inflammation, cell death, or lipogenesis--is involved in adipose tissue loss in cancer cachexia. Cancer 2008;113:1695-704. [Crossref] [PubMed]
- Dahlman I, Mejhert N, Linder K, et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer 2010;102:1541-8. [Crossref] [PubMed]
- Das SK, Eder S, Schauer S, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011;333:233-8. [Crossref] [PubMed]
- Shaw JH, Wolfe RR. Fatty acid and glycerol kinetics in septic patients and in patients with gastrointestinal cancer. The response to glucose infusion and parenteral feeding. Ann Surg 1987;205:368-76. [Crossref] [PubMed]
- Thompson MP, Cooper ST, Parry BR, et al. Increased expression of the mRNA for hormone-sensitive lipase in adipose tissue of cancer patients. Biochim Biophys Acta 1993;1180:236-42. [Crossref] [PubMed]
- Legaspi A, Jeevanandam M, Starnes HF Jr, et al. Whole body lipid and energy metabolism in the cancer patient. Metabolism 1987;36:958-63. [Crossref] [PubMed]
- Gercel-Taylor C, Doering DL, Kraemer FB, et al. Aberrations in normal systemic lipid metabolism in ovarian cancer patients. Gynecol Oncol 1996;60:35-41. [Crossref] [PubMed]
- Argilés JM, Busquets S, Lopez-Soriano FJ. Anti-inflammatory therapies in cancer cachexia. Eur J Pharmacol 2011;668 Suppl 1:S81-86. [Crossref] [PubMed]
- Mantovani G, Maccio A, Mura L, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med (Berl) 2000;78:554-61. [Crossref] [PubMed]
- Torti FM, Dieckmann B, Beutler B, et al. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 1985;229:867-9. [Crossref] [PubMed]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153-66. [Crossref] [PubMed]
- Hirai K, Hussey HJ, Barber MD, et al. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998;58:2359-65. [PubMed]
- Bing C, Bao Y, Jenkins J, et al. Zinc-alpha 2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A 2004;101:2500-5. [Crossref] [PubMed]
- Nordström EA, Ryden M, Backlund EC, et al. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes 2005;54:1726-34. [Crossref] [PubMed]
- Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol 1986;111:82-5. [Crossref] [PubMed]
- Fueger BJ, Czernin J, Hildebrandt I, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 2006;47:999-1006. [PubMed]
- Lee P, Greenfield JR, Ho KK, et al. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010;299:E601-606. [Crossref] [PubMed]
- Tsoli M, Moore M, Burg D, et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res 2012;72:4372-82. [Crossref] [PubMed]
- Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304-16. [Crossref] [PubMed]
- Batista ML Jr, Neves RX, Peres SB, et al. Heterogeneous time-dependent response of adipose tissue during the development of cancer cachexia. The J Endocrinol 2012;215:363-73. [Crossref] [PubMed]
- Lanza-Jacoby S, Lansey SC, Miller EE, et al. Sequential changes in the activities of lipoprotein lipase and lipogenic enzymes during tumor growth in rats. Cancer Res 1984;44:5062-7. [PubMed]
- Notarnicola M, Miccolis A, Tutino V, et al. Low levels of lipogenic enzymes in peritumoral adipose tissue of colorectal cancer patients. Lipids 2012;47:59-63. [Crossref] [PubMed]
- López-Soriano J, Argiles JM, Lopez-Soriano FJ. Lipid metabolism in rats bearing the Yoshida AH-130 ascites hepatoma. Mol Cell Biochem 1996;165:17-23. [Crossref] [PubMed]
- López-Soriano J, Argiles JM, Lopez-Soriano FJ. Sequential changes in lipoprotein lipase activity and lipaemia induced by the Yoshida AH-130 ascites hepatoma in rats. Cancer Lett 1997;116:159-65. [Crossref] [PubMed]
- Lopez-Soriano J, Argiles JM, Lopez-Soriano FJ. Marked hyperlipidaemia in rats bearing the Yoshida AH-130 ascites hepatoma. Biochem Soc Trans 1995;23:492S. [Crossref] [PubMed]
- Thompson MP, Koons JE, Tan ET, et al. Modified lipoprotein lipase activities, rates of lipogenesis, and lipolysis as factors leading to lipid depletion in C57BL mice bearing the preputial gland tumor, ESR-586. Cancer Res 1981;41:3228-32. [PubMed]
- Chen SZ, Qiu ZG. Combined treatment with GH, insulin, and indomethacin alleviates cancer cachexia in a mouse model. J Endocrinol 2011;208:131-6. [Crossref] [PubMed]
- Oliff A, Defeo-Jones D, Boyer M, et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 1987;50:555-63. [Crossref] [PubMed]
- Sherry BA, Gelin J, Fong Y, et al. Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J 1989;3:1956-62. [Crossref] [PubMed]
- Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 2006;17:314-20. [Crossref] [PubMed]
- Jatoi A, Ritter HL, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010;68:234-9. [Crossref] [PubMed]
- Jatoi A, Dakhil SR, Nguyen PL, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007;110:1396-403. [Crossref] [PubMed]
- Strassmann G, Fong M, Kenney JS, et al. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992;89:1681-4. [Crossref] [PubMed]
- Weidle UH, Klostermann S, Eggle D, et al. Interleukin 6/interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer Genomics Proteomics 2010;7:287-302. [PubMed]
- Maltoni M, Fabbri L, Nanni O, et al. Serum levels of tumour necrosis factor alpha and other cytokines do not correlate with weight loss and anorexia in cancer patients. Support Care Cancer 1997;5:130-5. [Crossref] [PubMed]
- Staal-van den Brekel AJ, Dentener MA, et al. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995;13:2600-5. [Crossref] [PubMed]
- Inadera H, Nagai S, Dong HY, et al. Molecular analysis of lipid-depleting factor in a colon-26-inoculated cancer cachexia model. Int J Cancer 2002;101:37-45. [Crossref] [PubMed]
- Soda K, Kawakami M, Kashii A, et al. Manifestations of cancer cachexia induced by colon 26 adenocarcinoma are not fully ascribable to interleukin-6. Int J Cancer 1995;62:332-6. [Crossref] [PubMed]
- Albertus DL, Seder CW, Chen G, et al. AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. J Thorac Oncol 2008;3:1236-44. [Crossref] [PubMed]
- Hale LP, Price DT, Sanchez LM, et al. Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res 2001;7:846-53. [PubMed]
- Díez-Itza I, Sanchez LM, Allende MT, et al. Zn-alpha 2-glycoprotein levels in breast cancer cytosols and correlation with clinical, histological and biochemical parameters. Eur J Cancer 1993;29A:1256-60. [Crossref] [PubMed]
- Todorov PT, McDevitt TM, Meyer DJ, et al. Purification and characterization of a tumor lipid-mobilizing factor. Cancer Res 1998;58:2353-8. [PubMed]
- McDevitt TM, Todorov PT, Beck SA, et al. Purification and characterization of a lipid-mobilizing factor associated with cachexia-inducing tumors in mice and humans. Cancer Res 1995;55:1458-63. [PubMed]
- Russell ST, Zimmerman TP, Domin BA, et al. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim Biophys Acta 2004;1636:59-68. [Crossref] [PubMed]
- Russell ST, Tisdale MJ. Effect of eicosapentaenoic acid (EPA) on expression of a lipid mobilizing factor in adipose tissue in cancer cachexia. Prostaglandins Leukot Essent Fatty Acids 2005;72:409-14. [Crossref] [PubMed]
- Rydén M, Agustsson T, Andersson J, et al. Adipose zinc-alpha2-glycoprotein is a catabolic marker in cancer and noncancerous states. J Intern Med 2012;271:414-20. [Crossref] [PubMed]
- Bing C, Mracek T, Gao D, et al. Zinc-alpha2-glycoprotein: an adipokine modulator of body fat mass? Int J Obes (Lond) 2010;34:1559-65. [Crossref] [PubMed]
- Russell ST, Tisdale MJ. The role of glucocorticoids in the induction of zinc-alpha2-glycoprotein expression in adipose tissue in cancer cachexia. Br J Cancer 2005;92:876-81. [Crossref] [PubMed]
- Gong FY, Deng JY, Zhu HJ, et al. Fatty acid synthase and hormone-sensitive lipase expression in liver are involved in zinc-alpha2-glycoprotein-induced body fat loss in obese mice. Chin Med Sci J 2010;25:169-75. [Crossref] [PubMed]
- Knapp ML, al-Sheibani S, Riches PG, et al. Hormonal factors associated with weight loss in patients with advanced breast cancer. Ann Clin Biochem 1991;28:480-6. [Crossref] [PubMed]
- Bouyekhf M, Rule DC, Hu CY. Effect of Catecholamines on Lipolysis and Esterification Invitro in Adipose-Tissue of Sheep Fed Low and High-Energy Diets. J Nutr Biochem 1993;4:80-5. [Crossref]
- Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153-64. [Crossref] [PubMed]
- Waldén TB, Hansen IR, Timmons JA, et al. Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. Am J Physiol Endocrinol Metab 2012;302:E19-31. [Crossref] [PubMed]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277-359. [Crossref] [PubMed]
- Klein S, Wolfe RR. Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J Clin Invest 1990;86:1403-8. [Crossref] [PubMed]
- Langin D, Portillo MP, Saulnier-Blache JS, et al. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol 1991;199:291-301. [Crossref] [PubMed]
- Susulic VS, Frederich RC, Lawitts J, et al. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem 1995;270:29483-92. [Crossref] [PubMed]
- Grujic D, Susulic VS, Harper ME, et al. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem 1997;272:17686-93. [Crossref] [PubMed]
- Cao L, Choi EY, Liu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011;14:324-38. [Crossref] [PubMed]


