
A longitudinal cohort study of symptoms and other concerns among Nigerian people with stages 3–5 chronic kidney diseases: study protocol
Introduction
There is a growing concern about the increasing burden of life-threatening conditions and the lack of services to address the needs of those affected in developing countries (1). In 2015, about 35.5 million people experienced serious health-related suffering due to life-threatening and life-limiting conditions. A disproportionate number (more than 80%) of these individuals live in low-income and middle-income countries with severely limited access to any palliative care (2).
Chronic kidney disease (CKD) (defined with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min) is a life-threatening condition of particular concern because of the worsening trend in its prevalence. For instance, the prevalence of CKD in Nigeria is 12.1% (3), and similar to a pooled prevalence rate of 13.9% reported in a meta-analysis of 21 sub-Saharan African (SSA) studies (4,5). Future estimates project an exponential increase in the burden of CKD, and that developing countries will account for up to 70% of people with CKD worldwide by 2030 (6). An increase in the burden and service needs of people with CKD in SSA also seems inevitable given a projection of up to 130% increase in the prevalence of diabetes mellitus and hypertension, (two leading contributors to CKD burden) in Africa by the year 2020 (7). Beyond impacting on dialysis and renal replacement services, increasing prevalence of CKD may significantly increase the need for palliative care. This is due to disease progression, deterioration in organ function, accumulation of toxic products, high symptom load, co-morbidity of other life-threatening illnesses, and high mortality, thereby necessitating end of life-care (8,9).
Palliative care is defined as an approach that improves the quality of life of patients and their families facing life-threatening illness (1). The current evidence highlights the growing need for physicians to pay attention to the palliative or end-of-life care needs of patients with CKD as they have a shortened life expectancy (10,11). This is because patients with CKD often experience high symptom burden (10) including pain, fatigue, itching and sleep problems, all contributing to an overall negative impact on the patients’ quality of life (QoL) (12). To this end, the American Society of Nephrologists recommended integration of palliative care into treatment of patients with CKD (13).
Several factors are thought to drive the increasing burden of CKD as well as existing gaps in palliative care services. Such factors include high prevalence of chronic illnesses, absence of kidney disease prevention programs, inequitable distribution of resources, low literacy, late presentation and lack of governmental funding necessitating out-of-pocket payments among others (14-16). In addition to these factors, both psychosocial and biomedical factors often impact treatment decision, the course of the illness, palliative care needs, and quality of life in CKD (16,17). Previous studies have reported high rates of a range of psychosocial factors, including anxiety, depressive disorders, poor social support and suicidality among patients with CKD (18), with rates of depression and anxiety symptomatology reaching up to 33.4% (16,19,20). These psychosocial factors often have far reaching adverse consequences on patients’ function, illness course, outcome and palliative care needs (16,21-23). In line with the World Health Organisation definition, we need all these dimensions to be measured to truly understand the extent of need (1).
The extent of palliative care needs and the specific roles of biopsychosocial factors (including social support, diet, hypertension, and diabetes mellitus among other) on CKD in SSA are largely unknown because evidence is scarce (24,25). The extrapolation of findings from existing literature to SSA population is limited because these studies were mostly from developed countries and focused on advanced CKD. Again, access to palliative care services and available resources in SSA are very poor (2) despite the call for palliative care for all in the new universal health coverage goals (26). Hence more data from valid measures are needed to understand palliative care-related problems, design policy and plan palliative care services in line with the recent World Health Assembly resolution on palliative care (27). There is also a need for broader inclusion of early stages of CKD as proposed in this study to enhance early integration of palliative care as recommended by the World Health Organization. Despite the weight of evidence, there is lack of focus on palliative care in the global health literature and more research is needed to underpin public health approaches to expansion in SSA (28,29). In light of the above, this longitudinal study aims to measure the prevalence and bio-psychosocial factors associated with palliative care symptoms and concerns among people diagnosed with stages 3–5 CKD. The specific objectives are:
- To measure the prevalence of symptoms and other concerns;
- To identify the bio-psychosocial factors associated with palliative care-related symptoms concerns among people with stages 3–5 CKD;
- To determine whether there is change in outcomes over a 3-month period;
- To measure the use and identify predictors of formal and informal healthcare services during this time.
Methods
Study design and setting
This is a multi-centre longitudinal observational study among people diagnosed with CKD, collecting self-report data at baseline (T0) and 3-month follow-up in the renal outpatient clinics of two tertiary teaching hospitals in Lagos, Nigeria. These study centres are government-funded hospitals within Lagos State which has a population of about 20 million people. The hospitals also serve people from neighbouring states, other parts of Nigeria and nearby African countries. The two hospitals have nephrology units under their respective medical departments with facilities for renal replacement therapy (RRT) including renal dialysis.
Study participants
Inclusion and exclusion criteria: The eligibility criteria include adults 18 years and above with confirmed diagnosis of stages 3–5 CKD base on eGFR of <60 mL/min (30). The diagnosis of CKD is made by consultant Nephrologists (in centres where study is ongoing) based on evidence of kidney damage for at least 3 months and/or glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 for at least 3 months with or without kidney damage (31). Those deemed by their treating clinicians to be too unwell to participate in a research interview with study instruments were excluded from recruitment.
Sample size calculation
We determined the sample size to estimate prevalence of palliative care symptoms and concerns among people with stages 3–5 CKD. This gave the largest sample size estimate and adequate to meet the other study objectives. The margin of error was set at 0.5%, the confidence interval was set at 95%, and response distribution was set at 50% (32). The population size was derived from the average number of new patients with CKD seen in the study sites annually, which is estimated from clinic records as n=520. Of these, half or lesser are diagnosed with stage 3–5 CKD among the new patients. Thus, an estimated sample size of 156 participants was derived, and we aim to recruit additional 20% to allow for attrition during follow-up resulting in sample size of 187 participants. We consider the sample size (n=187) ideal as it is larger than the estimate derived from other study objectives. For instance, a sample size of 100 was needed to detect small effect size of 0.3 (8) for change in palliative care concerns of people with stage 3–5 CKD over a 3-month period (objective 3) with alpha =0.05, power =0.95, one group and two data point measure using repeated measure ANOVA for within group comparison (33).
Data collection
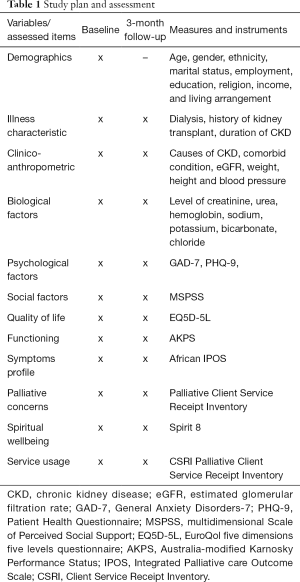
A single pack containing the tools for data collection is created for baseline (T0) and another set for follow-up at 3-month (T1) for each patient. The pages are colour coded by time point, and preceded by a log page to complete the interview dates and a front cover with the patient’s ID number. Data is collected at baseline with all the tools and subsequently a repeat follow-up assessment at 3 months excluding the demographic chart (see Table 1). A flexibility of 4 weeks before and after 3 months for the second data collection is allowed. This is to accommodate rescheduling of participants 3 months appointments. The follow-up data collection at 3 months is set to coincide with the 3-monthly schedule of follow-up appointments in the study centres. To reduce follow-up attrition, respondents are sent text messages 1 month after the baseline assessment to remind them of the study and confirm date for follow-up appointment. Additionally, participants are rung to remind them of the follow up data collection appointment. In cases of loss to follow-up, reasons for attrition are documented if known to the researcher. This information will help to estimate response rate at the end of the project.
Full table
Data are collected by trained research assistants. The study participants are recruited consecutively from the outpatient clinics and data collection are supervised by the investigators. While some of the measures are self-reported, others are interviewer administered according to standard described in the original scales. The study participants are interviewed in separate consulting room to ensure confidentiality of responses. All questionnaire is de-identified and locked up in a safe cabinet where only the principal investigators have access after completion of data collection.
Ethical approval
The study protocol was approved by the Health and Research Ethical Committee of the Lagos University Teaching Hospital (approval number: ADM/DCST/HREC/APP/1953), Lagos State University Teaching Hospital (approval number: LREC./10/1001) and Kings College London (approval number: HR-17/18-5413). All eligible participants were explained all study details in writing and in person before giving informed consent. Participants are enrolled and interviewed following written informed consent is obtained. Data are stored in a research database in accordance with the Standard Protection Authority.
Study instruments
Assessments are done at baseline and a follow-up at 3 months using the following interviewer administered and self-report measures.
The interviewer administered measures include:
- Study-specific questionnaire: this was designed to elicit socio demographic data including age, gender, employment status, occupation, religion, ethnicity, level of education, and marital status. In addition, clinical history is elicited from patients and their clinical records on a range of variables including duration of CKD/illness, etiological risk of CKD (including hypertension, and diabetes mellitus), average number of dialysis, co-morbidities, reasons why they have not had a renal transplant, past personal and history of emotional problems in family members. Some important clinical variables such as medication use, the average sessions of dialysis per week are extracted from patient’s case notes. Other clinical/biomedical variables are derived from results of standardized investigations or measurement carried out. Some of these variables include body mass index (BMI), fasting blood sugar/2-hour post prandial, urinalysis, blood pressure, E/UR/Cr, and the primary cause of disease is extracted from the case note if documented.
- Australia-modified Karnosky Performance Status (AKPS): the AKPS was designed as a useful modification of the Karnofsky Performance Status (KPS) by combining the KPS which is the gold standard in measurement of functioning in clinical settings and the Thorne-modified KPS (TKPS) which is focuses on community-based care. The AKPS was designed as a measure considered to be more appropriate for any care setting and especially in outpatient populations (34). It is a measure of the patient’s overall performance status or ability to perform their activities of daily living. It is an ordered categorical scale with 11 levels and scores are assigned in increments of 10 between 0 and 100. The scores are assigned by a clinician based on observations of a patient’s ability to perform common tasks relating to activity, work and self-care and in between scores (e.g., 35) are invalid. Decreasing numbers indicate a reduced performance status. A score of 100 signifies normal physical abilities with no evidence of disease, while a score of 0 refers to a dead/deceased patient. It can be completed in less than 2 minutes (35).
- The African Palliative Care Association (APCA) African Palliative Outcome Scale (APOS): the APOS is an interviewer administered instrument that was designed using/adapted from the Integrated Palliative care Outcome Scale (IPOS) for use among Africans (36). It was developed for use in patients with life threatening conditions to improve outcome by assessing the full range of needs of patients with life-threatening conditions, thereby ensuring that patients and family caregivers get the best possible care. It contains ten questions on physical and psychological symptoms, spiritual, practical and emotional concerns and psychosocial needs of the patient and their relatives. The first seven questions concern the patients while the remaining three questions are directed at family and other informal caregivers. It has been used mainly in east and Southern African countries including Botswana, Kenya, Malawi, South Africa, Tanzania, Uganda, Zambia and Zimbabwe (37). The scale is simple to administer, Cronbach’s Alpha was 0.6 indicating expected moderate internal consistency; test-retest found high intra-class correlation coefficients for all items (0.78–0.89), and can be completed within an average time of 8 to 9 minutes (37).
- Client Service Receipt Inventory (CSRI): the CSRI was designed about three decades ago to collect information on service utilisation, income, accommodation and other cost-related variables. There have been several versions designed for use in various patient/client populations including Generic mental health CSRI, chronic pain CSRI, Arthritis study CSRI and Homestart CSRI among others (38). For this study, we adapted the modified Palliative version CSRI questionnaire, which has been adapted for African population (39).
- Spirit-8: the Spirit 8 was designed to measure spiritual well-being (SWB) among palliative care patients in SSA as a means to improve spiritual care (40). It was developed from the Missoula Vitas Quality of Life Index (MVQOLI), a tool created in the USA (41). The scale composes of eight items that are scored on a 5-point Likert scale from 1 (worst) to 5 (best). The instrument is interpreted by summing the individual scores from all the 8 items resulting in a possible score of between 8 and 40 with higher scores indicating a better outcome. It has been used in African populations and shown to have good internal consistency (Cronbach’s alpha =0.73) (40).
The self-reported measures include:
- Patient Health Questionnaire (PHQ-9): the Patient Health Questionnaire (PHQ-9) is used in this study to screen for depression. It is a 9-item self-report questionnaire that efficiently establishes DSM-IV criteria based psychiatric diagnoses (42). PHQ-9 is a validated and shown to have good psychometric properties (internal consistency =0.85 and test-retest reliability, r=0.894, P<0.001). Respondents are required to rate how they feel in the preceding 2 weeks. Each question is scored 0 to 3 (0 = not at all, 1 = several days, 2 = more than half the days and 3 = early everyday) with a resulting range of 0 to 27. The cut-off scores of 5, 10 and 15 are suggestive of mild, moderate and severe depression respectively (42,43). Adewuya et al. (43) reported that a cut-off of 5 or higher provides a sensitivity of 89% and specificity of 98% among Nigerian university students.
- General Anxiety Disorders-7 (GAD-7): the GAD-7 is a 7-item questionnaire developed to identify cases of GAD and measure the severity of GAD symptoms (44). The GAD-7 asks participants to rate how often they have been bothered by each of the 7 core symptoms included in the questionnaire over the preceding 2 weeks. Response categories include “not at all”, “several days”, “more than half the days”, and “nearly every day”, scored as 0, 1, 2, and 3, respectively with the total score ranging from zero to 21. A cut-off score of 10 or higher (recommended cut off score) provides a sensitivity of 89% and a specificity of 82% for GAD (45).
- Multidimensional Scale of Perceived Social Support (MSPSS): the MSPSS is used to measure perceived social support in this study (46). It is a 12-item instrument that provides assessment of three sources of support: family support, friends support, and significant others support. It is scored on a 5-point Likert scale with scores ranging from 1 “strongly disagree” to 5 “strongly agree”. Items 3, 4, 8 and 11 measure family support; items 6, 7, 9 and 12 measures friend support while items 1, 2, 5, and 10 measures significant other support. It has been shown to have good psychometric properties with a Cronbach’s alpha of 0.82 and well used in previous studies in this environment (47-49).
- EuroQol five dimensions five levels questionnaire (EQ5D-5L): the EQ-5D (50) is a disease non-specific (generic) instrument that measures quality of life across several cultural setting and validated in African population (51). The EQ-5D is brief, with five questions that bother on mobility, self-care, pain, usual activities and psychological status as well as a visual analogue scale which asks patients to state their opinion of their state of health as of the day of assessment ranging from 0 (worst imaginable state) to 100 (best imaginable). EQ5D-5L which has five levels of severity (no problems, slight problems, moderate problems, severe problems, and extreme problems). The EQ5D-5L is used in this study because of its superior discriminating effects compared to the EQ5D-3L among patients with other chronic medical conditions (52).
Data analyses plan
The study data are longitudinal quantitative in nature, and will be entered into predesigned Excel spreadsheets. This will be exported to IBM-SPSS-22 for analysis, including creation of graphs, descriptive statistics, univariate analysis and multiple regression.
The primary outcomes (dependent variables) including palliative care-related symptoms and concerns, spiritual wellbeing and quality of life are the summary scores derived from APOS, AKPS; EQ-5D and spirit-8 respectively. The secondary measures of interest [independent variables] include psychosocial factors (such as anxiety, depression, and social support) and biomedical factors including levels of electrolyte, creatinine and urea, eGFR, blood pressure, blood sugar level, and BMI. Analyses plans for the main study objectives are outlined below.
Study objective 1: assess the prevalence of symptoms and other concerns among people with stages 3–5 CKD
Socio demographic, baseline clinical characteristics, prevalence and distribution of scores on palliative care symptoms and concerns will be summarized with descriptive statistics using frequencies, means (SD) and percentages after testing for normality. Non-parametric data will be summarized with median and interquartile range. A P value of 0.05 will be used to determine significance in all statistical tests except for the decision to include variables in multivariate analysis, when 0.1 will be used. For univariate analysis of demographic/clinical characteristics with primary outcomes at baseline, ANOVA will be used for ordinal variables and linear regression for continuous.
Study objective 2: determine the bio-psychosocial factors associated with symptoms and other concerns among people with stages 3–5 CKD
Regression analysis will be conducted to investigate whether or not bio-psychosocial factors predict the experience of symptoms and concerns among participants following univariate correlation analyses. For regression analyses, the relationship of each primary outcome (palliative care symptoms/concerns, spiritual wellbeing and quality of life) will be explored with the secondary measures of interest including psychosocial factors (such as anxiety, depression, and social support), and biomedical factors (including electrolytes, creatinine and urea, eGFR, blood pressure, blood sugar, and BMI) extracted from the case notes.
Study objective 3: investigate any change in the symptoms and other concerns of people with stages 3–5 CKD over a 3-month period
Changes in symptoms and other concerns over a 3-month period are analysed using repeated measure ANOVA. Longitudinal multilevel modelling is an approach which makes the most efficient use of data collected over time. Thus, longitudinal multilevel modelling will be conducted to evaluate the contribution of biopsychosocial variables to the variance of change in palliative care symptoms, concerns, spiritual wellbeing and quality of life over the two data points (baseline to follow up at 3 months).
Study objective 4: assess the use of formal and informal healthcare services during this time
The components of care recorded in the CSRI will be grouped into care themes according to the issues they address and the way in which they are delivered. Descriptive statistics is used to summarize the use of formal and informal healthcare services. Univariate-correlation analyses will be performed to investigate the relationship between health care service usage and sociodemographic and clinical factors.
Discussion
This is the first study to investigate symptoms and other concerns among people with stages 3–5 CKD in Africa despite indications for palliative care services in CKD. We believe there are several elements in the design and implementation of this study that allow advantages for quality data to inform the care of CKD in SSA.
First, this study will provide substantive data in a neglected region on the symptom burden and services in CKD, in order to improve care for people with CKD. Our goal is early integration of palliative care services into management of CKD and to direct future clinical studies. Second, the study will generate health outcome and cost data that is Africa-specific and multidimensional in line with the palliative care definition and advancing knowledge beyond what is known already. Third, findings from this work can inform stakeholders and guide policy to design palliative care services to support patients with CKD. By extension, findings from this study may be beneficial to patients with other life-threatening conditions, and influence incorporation of palliative care training for professionals involved in the care of patients with CKD and other life-threatening conditions. We plan to use data from this study to drive advocacy for evidence-based interventions to mitigate the burden of CKD and advance the global science of conservative management of CKD in SSA. To enhance translation, study findings will be disseminated in peer reviewed journal and presentations in conferences and meetings. Fourth, the staffs working on the project are indigenous African researchers, and considerable capacity has been built through ongoing training and the conduct of this multisite study. In addition, the nature of this project can promote intentional collaboration between African and UK expertise for future multi-national studies. Lastly, the novel method in this study brings together an integrated mixture of outcome measurement, biopsychosocial factors, longitudinal evaluation, health economics and service usage to evaluate palliative concerns in CKD.
The careful attention to multi-professional inclusivity and consultation has maximised the feasibility of this study where many logistical challenges are possible. We believe that the presentation of this protocol offers greater transparency in the aims, objectives and analysis of our study, and will allow greater quality reporting of our large dataset.
In conclusion, this study is a novel approach utilizing relevant measures to collect comprehensive clinical, biological and psychosocial data to improve the understanding of CKD in relation to patients and caregiver’s experiences, their symptom and other concerns and service usage. The BUILD care team welcomes collaborations with both national and international investigators.
Acknowledgements
We are grateful to the BUILD Care African Project and Kings College London for providing funding for this project. The authors would like to thank the Provost and staff of the College of Medicine, University of Lagos for various support provided for this work. Finally, we are grateful to the staff and patients at the participating facilities.
This study is funded by the BUILD Care African Project from Kings College London.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was designed and is being conducted according to the latest version of the Declaration of Helsinki. The Institutional Review Boards of the Lagos University Teaching Hospital (approval number: ADM/DCST/HREC/APP/1953), Lagos State Teaching Hospital (approval number: LREC./10/1001) and Kings College London (approval number: HR-17/18-5413) gave ethical approval to study design, content and recruitment plans. Written informed consent is obtained from all enrolled participants and their caregivers after they had the opportunity to ask all open questions and received a satisfying answer from the study researcher.
References
- World Health Organisation. Planning and implementing palliative care services: a guide for programme managers. Geneva: World Health Organization, 2016.
- Knaul FM, Bhadelia A, Rodriguez NM, et al. The Lancet Commission on Palliative Care and Pain Relief-findings, recommendations, and future directions. Lancet Glob Health 2018;6:S5-6. [Crossref]
- Odubanjo MO, Oluwasola AO, Kadiri S. The epidemiology of end-stage renal disease in Nigeria: the way forward. Int Urol Nephrol 2011;43:785-92. [Crossref] [PubMed]
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038-47. [Crossref] [PubMed]
- Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e174-81. [Crossref] [PubMed]
- Kane PM, Vinen K, Murtagh FE. Palliative care for advanced renal disease: a summary of the evidence and future direction. Palliat Med 2013;27:817-21. [Crossref] [PubMed]
- Schena FP. Epidemiology of end-stage renal disease: International comparisons of renal replacement therapy. Kidney Int 2000;57:S39-45. [Crossref]
- Murtagh FE, Addington-Hall JM, Higginson IJ. End-stage renal disease: a new trajectory of functional decline in the last year of life. J Am Geriatr Soc 2011;59:304-8. [Crossref] [PubMed]
- Smith C, Da Silva-Gane M, Chandna S, et al. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 2003;95:c40-6. [Crossref] [PubMed]
- Moss AH. Palliative Care in Patients with Kidney Disease and Cancer. Onco-Nephrology Curriculum. USA: American Society of Nephrology, 2016.
- Turin TC, Tonelli M, Manns BJ, et al. Chronic kidney disease and life expectancy. Nephrol Dial Transplant 2012;27:3182-6. [Crossref] [PubMed]
- Kimmel PL, Emont SL, Newmann JM, et al. ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 2003;42:713-21. [Crossref] [PubMed]
- Renal Physicians Association and American Society of Nephrology. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis Washington DC: American Society of Nephrology, 2000.
- Adejumo OA, Akinbodewa AA, Okaka EI, et al. Chronic kidney disease in Nigeria: Late presentation is still the norm. Niger Med J 2016;57:185-9. [Crossref] [PubMed]
- Arogundade FA, Zayed B, Daba M, et al. Correlation between Karnofsky Performance Status Scale and Short-Form Health Survey in patients on maintenance hemodialysis. J Natl Med Assoc 2004;96:1661-7. [PubMed]
- Olagunju AT, Campbell EA, Adeyemi JD. Interplay of anxiety and depression with quality of life in endstage renal disease. Psychosomatics 2015;56:67-77. [Crossref] [PubMed]
- Campbell EA, Olagunju AT, Oyatokun BO, et al. Psychiatric Morbidity among individuals with End Stage Renal Disease in Nigeria. Nig Q J Hosp Med 2014;24:189-94.
- De Sousa A. Psychiatric issues in renal failure and dialysis. Indian J Nephrol 2008;18:47-50. [Crossref] [PubMed]
- Assefa B, Duko B, Ayano G, et al. Prevalence and Factors Associated with Depressive Symptoms among Patient with Chronic Kidney Disease (CKD) in Black Lion Specialized Hospital and Saint Paulo’s Hospital Millennium Medical College, Addis Ababa, Ethiopia: Cross Sectional Study. J Psychiatry 2016;19:390. [Crossref]
- Chen CK, Tsai YC, Hsu HJ, et al. Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics 2010;51:528-528.e6. [Crossref] [PubMed]
- Bristowe K, Horsley HL, Shepherd K, et al. Thinking ahead--the need for early Advance Care Planning for people on haemodialysis: A qualitative interview study. Palliat Med 2015;29:443-50. [Crossref] [PubMed]
- Lowney AC, Myles HT, Bristowe K, et al. Understanding What Influences the Health-Related Quality of Life of Hemodialysis Patients: A Collaborative Study in England and Ireland. J Pain Symptom Manage 2015;50:778-85. [Crossref] [PubMed]
- Moreira JM, da Matta SM, Melo e Kummer A, et al. Neuropsychiatric disorders and renal diseases: an update. J Bras Nefrol 2014;36:396-400. [Crossref] [PubMed]
- Bates MJ, Chitani A, Dreyer G. Palliative care needs of patients living with end-stage kidney disease not treated with renal replacement therapy: An exploratory qualitative study from Blantyre, Malawi. Afr J Prim Health Care Fam Med 2017;9:e1-6. [Crossref] [PubMed]
- Collins ES, Witt J, Bausewein C, et al. A Systematic Review of the Use of the Palliative Care Outcome Scale and the Support Team Assessment Schedule in Palliative Care. J Pain Symptom Manage 2015;50:842-53.e19. [Crossref] [PubMed]
- World Health Organisation. WHA67.19 - Strengthening of Palliative Care as a Component of Comprehensive Care Throughout the Life Course. WHA Resolution; Sixty-seventh World Health Assembly 2014 (English version). 2014.
- World Health Organisation. What is universal coverage? 2013.
- Harding R, Higginson IJ. Inclusion of end-of-life care in the global health agenda. Lancet Glob Health 2014;2:e375-6. [Crossref] [PubMed]
- Harding R, Selman L, Powell RA, et al. Research into palliative care in sub-Saharan Africa. Lancet Oncol 2013;14:e183-8. [Crossref] [PubMed]
- Azegbeobor J, Lasebikan VO. Depression and Disability in Chronic Kidney Disease in Nigeria: A Case-Control Study. Int Neuropsychiatr Dis J 2016;7:1-13. [Crossref]
- Kidney Disease: Improving Global Outcomes (KDIGO). Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19-62. [Crossref]
- Raosoft. Inc. Sample Size Calculator Raosoft, Inc.; 2004.
- Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91. [Crossref] [PubMed]
- Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice BMC Palliat Care 2005;4:7. [ISRCTN81117481]. [Crossref] [PubMed]
- Witt J, Murtagh F, De Wolf-Linder S, et al. Introducing the Outcome Assessment and Complexity Collaborative (OACC) Suite of Measures A Brief Introduction. 2013. Available online: https://www.kcl.ac.uk/lsm/research/divisions/cicelysaunders/attachments/Studies-OACC-Brief-Introduction-Booklet.pdf. Accessed June 2017.
- Dix O. Impact of the APCA African Palliative care Outcome Scale (POS) on care and practice. 2012. Available online: https://www.kcl.ac.uk/lsm/research/divisions/cicelysaunders/attachments/Impact-of-the-APCA-African-Palliative-care-Outcome-Scale-(POS)-on-care-and-practice.pdf. Accessed January 23 2018.
- Harding R, Selman L, Agupio G, et al. Validation of a core outcome measure for palliative care in Africa: the APCA African Palliative Outcome Scale. Health Qual Life Outcomes 2010;8:10. [Crossref] [PubMed]
- PSSRU, Blogs. Client Service Receipt Inventory. 2017. Available online: http://www.pssru.ac.uk/blogs/csri/. Accessed June 7 2017.
- Lowther K, Simms V, Selman L, et al. Treatment outcomes in palliative care: the TOPCare study. A mixed methods phase III randomised controlled trial to assess the effectiveness of a nurse-led palliative care intervention for HIV positive patients on antiretroviral therapy. BMC Infect Dis 2012;12:288. [Crossref] [PubMed]
- Selman L, Siegert RJ, Higginson IJ, et al. The "Spirit 8" successfully captured spiritual well-being in African palliative care: factor and Rasch analysis. J Clin Epidemiol 2012;65:434-43. [Crossref] [PubMed]
- Byock IR, Merriman MP. Measuring quality of life for patients with terminal illness: the Missoula-VITAS quality of life index. Palliat Med 1998;12:231-44. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [Crossref] [PubMed]
- Adewuya AO, Ola BA, Afolabi OO. Validity of the patient health questionnaire (PHQ-9) as a screening tool for depression amongst Nigerian university students. J Affect Disord 2006;96:89-93. [Crossref] [PubMed]
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. [Crossref] [PubMed]
- Adewuya AO, Ola BA, Coker OA, et al. Prevalence and associated factors for suicidal ideation in the Lagos State Mental Health Survey, Nigeria. BJPsych Open 2016;2:385-9. [Crossref] [PubMed]
- Zimet GD, Powell SS, Farley GK, et al. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 1990;55:610-7. [Crossref] [PubMed]
- Onyishi IE, Okongwu OE, Ugwu FO. Personality and Social Support as predictors of life satisfaction of Nigerian Prisons Officers. Eur Sci J 2012;8:110-25.
- Olagunju AT, Olutoki MO, Ogunnubi OP, et al. Late-life depression: Burden, severity and relationship with social support dimensions in a West African community. Arch Gerontol Geriatr 2015;61:240-6. [Crossref] [PubMed]
- Olutoki MO, Olagunju AT, Adeyemi JD. Correlates of depressive illness among the elderly in a mixed urban community in Lagos, Nigeria. Aging Ment Health 2014;18:561-9. [Crossref] [PubMed]
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [Crossref] [PubMed]
- Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. [Crossref] [PubMed]
- Wang P, Luo N, Tai ES, et al. The EQ-5D-5L is More Discriminative Than the EQ-5D-3L in Patients with Diabetes in Singapore. Value Health Reg Issues 2016;9:57-62. [Crossref] [PubMed]


