Outcome measurement and complex physical, psychosocial and spiritual experiences of death and dying
Introduction
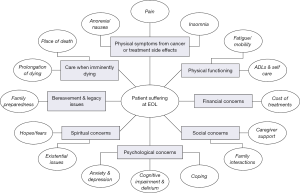
Patients with advanced illnesses, especially near the end of life, often experience multiple complex symptoms (1). These include, but are not limited to, physical symptoms like pain, fatigue, nausea, drowsiness, shortness of breath, but also profound and often complex psychological, social and spiritual components like delirium, depression (suicide risk), challenges around advance care planning, loss of autonomy and meaning in life, request for hastened death and spiritual or existential crisis (Figure 1) (1). Early and prompt recognition of these clinical problems is known to provide relief and better outcomes for these dying patients, their caregivers and family members (2). This is the essence of quality palliative care.
Often, symptoms of patients with advanced illnesses remain under recognized and not adequately managed (3,4). Measuring patient reported outcomes (PROs) has shown to improve awareness of unmet needs of patients and care-givers by allowing clinicians to address such issues in a timely manner, hence, leading to improvement in overall quality of life (QoL) of such patients (5-7). At the same time, it is important to keep in mind that patients with advanced illnesses especially near the end of life develop cognitive impairment like delirium (8) and hence, in those circumstances, are unable to provide a reliable account of symptom distress. During these times, the assessments may be performed by the family member, when available, and if not, then by the bedside nurse (9-11).
Table 1 highlights the issues, with an order of priority, which exist at the core of practice of palliative care both at a generalist and specialist care level. These core principles and elements of quality palliative care practice are also used during the process of accreditation of training programs in hospice and palliative care (12) and certification of palliative care programs (13) in United states, Canada and other countries. While multiple tools have been developed over the years to identify and measure the different outcomes experienced by the patients with advanced illnesses (14), earlier referral to palliative care teams can improve QoL of cancer patients (2,15-17) and these tools aid the professionals in this process.

Full table
Death, a physical experience!
It would be hard to progress into any depths of psycho-social and emotional assessments without timely identification and effective management of the distressing physical symptoms. Edmonton Symptom Assessment Scale (ESAS) (18-21) is one such tool which encompasses many such domains if included in the evaluation process. It is a psychometrically validated tool for regular assessment of symptom distress in advanced cancer patients in the palliative care setting around the world and translated into over 20 languages (19). Patients are asked to grade the severity of their symptoms on average from 0 (no symptom) to 10 (worst symptom) in the last 24 hours. ESAS has a high test-retest reliability (>0.8) and has been validated in many clinical settings including cancer patients. A very important aspect of using ESAS is the ease and convenience of use by self-reporting symptom outcomes in a relatively low time (22). The minimal clinically important difference (MCID) for ESAS scores is 1 point for individual score and 3–4 points for the sum of physical, emotional and ESAS total scores (23,24). ESAS physical score [0–60] is equal to sum of individual scores for pain, fatigue, nausea, drowsiness, shortness of breath and appetite (23).
Functional assessment
An important aspect of patient care involves on-going assessment of patient’s functional status. Loss of ability to perform activities independently has profound effects on patients’ emotional well-being and hence it is vital to assess this physical domain to highlight need for necessary support early on like preventing falls, provision of assistive devices like cane or walker and handicap parking placard. An easy and quick assessment tool for functional status is the Eastern Cooperative Oncology Group performance status (ECOG) (25,26). ECOG is used to assess patient’s functional abilities and then determine treatment options and overall prognosis. It ranges from “0”, fully active to “5”, death. Other tools to assess functional assessment include Karnofsky Performance Status (KPS) (25,27,28) and Palliative Performance Scale (PPS) (29,30). There remain inter-observer differences among these assessments (31-35).
Delirium screening
Delirium has been reported in approximately 60–90% of patients in the last weeks to hours before death (8,36,37). Approximately 50% to 70% of patients with delirium present with hyperactive or mixed subtypes, characterized by agitation, restlessness or violent behavior which can be highly distressing to patients, caregivers, and health care professionals, not to mention, sometimes with significant safety risks (38-40). Confusion Assessment Method (CAM) can be used as a quick screener for development of delirium (41). Memorial Delirium Assessment Scale (MDAS) (42), used in a more specialized setting, is highly correlated with existing measures of delirium and cognitive impairment. MDAS score of 7/30 yields the highest sensitivity 98% and specificity 97% for delirium diagnosis in cancer patients (43).
In case of altered mental status, patient reports may not be reliable. Under such circumstances reports from family care-givers and nurse (proxy) can provide useful information for timely and feasible interventions (9,10). It is ideal to use the caregiver account as there is better association noted between patient and care-giver ratings for symptom assessment as compared to patient and the clinician (11).
In addition, when validated tools do not exist, it is essential to review the PROs for a universal screening to better assist in management of complex issues faced by patients near end of life. These include:
Review of medications
This is an important aspect of palliative care evaluation. Effective review of polypharmacy is essential, especially in cases with concurrent use of opioids and psychoactive medications, to reduce the risk of complications like excessive sedation, delirium, falls, with resulting increase in psychosocial distress (44-49). Having a pharmacist in the interdisciplinary team facilitates this process (50).
Other physical symptoms
Effective assessment and management of symptoms like severe constipation, nausea and shortness of breath is essential to reduce the burden of overall distress (51,52).
In summary, addressing physical PROs early-on, allows clinicians to gain access into deeper and often much more vital aspects of patient distress, i.e., psychological, emotional and spiritual. Table 1 highlights the domains involved in delivery of palliative care close to end of life with emphasis on timeliness of management.
Death, a psychological experience!
Hospital Anxiety and Depression Scale (HADS)
Feelings of anxiety and depression are common among patients with advanced illnesses. These may be measurable by HADS (53,54). HADS is a 14-question self-assessment scale which asks of patients to mark the answer which comes closest to how they have been feeling in the past week. It is a reliable instrument for detecting states of anxiety and depression in the setting of an outpatient clinic.
Each item is scored from 0 to 3 which gives a score between 0 and 21 for either depression subscale or anxiety subscale. Cut-off point has been established as 8/21 for anxiety or depression which gives a specificity of 0.78 and a sensitivity of 0.9 for anxiety (HADS-A) and a specificity of 0.79 and a sensitivity of 0.83 for depression (HADS-D). Patients with scores between 8 and 10 indicate mild depression, scores between 11 and 14 indicate moderate depression, and scores between 15 and 21 indicate severe depression. Similar scores are considered for anxiety subscale. Patients with moderate to severe depression and/or anxiety should be referred to palliative care teams earlier for further evaluation, offered counseling services (55) and if found necessary, referred to a psychiatrist for detailed consultation and potential treatment. It can, however, be time consuming to use this tool in daily clinical settings and hence it is mostly used in the context of research.
ESAS (18,19)
Some elements of ESAS allow for assessment of the psychological symptoms, i.e., ESAS-anxiety and ESAS-depression, alone or together as ESAS-emotional score (sum of individual scores for depression and anxiety) (23,24), and also ESAS-well-being. Routine evaluation of these outcomes may assist clinicians in picking up often subtle presentations of these domains, which may otherwise go unnoticed and in fact contribute to overall high symptom expression.
Depression and suicidality
A single item question “Are your depressed?”, has shown to be a reliable clinical tool (56) with greater validity than other brief screening measures like visual analog scale for depressed mood (57) and the Beck Depression Inventory-Short Form (58-60).
There are no other formal and validated tools which are routinely used for measuring outcomes related to feelings of selflessness, worthlessness, loss of autonomy or meaning in life. Patient Dignity Question (PDQ) —“Is there anything else about you as a person that you would like us to know, in order to give you the best care possible?” also by Chochinov and colleagues (61) can be effective in exploring such issues and supporting personhood. “This Is ME (TIME)” questionnaire developed from PDQ can also be a starting point for such conversations, in specialized settings, when time allows (62). These preliminary studies aimed at eliciting personhood and enhancing dignity have been conducted in nursing home residents. Further validation of this seminal work in cancer and other non-cancer patients is justified.
Death, a social experience!
Assessment and documentation of the structure and function of the patient’s social network and support system is extremely important. It can aid the clinicians to aim for a goal of better outcomes for their patients.
Financial distress
Socio-economic status can have a vital impact on overall patient outcomes. Financial suffering may impact patient’s way of coping and compliance with treatment recommendations (63,64). Financial toxicity is a clinically relevant patient-centered outcome (65). COmprehensive Score for financial Toxicity (COST) (66) has been validated as reliable outcome measure, however, it is lengthier and time consuming. Financial distress is one of the two new variables (along with spiritual pain) added to the ESAS (18,19,22) and measured in a similar way like other ESAS dimensions on the 11-point scale from 0 to 10 (64,67), which can be used as a quick tool for assessment.
Care-givers, part of the social circle
Another important and often over-looked aspect is the care-givers and their well-being. Care-givers play an important role in the well-being of a patient and defining the goals of care. Vast majority of Hispanic patients in US and LA (over 80%) preferred family involvement in decision-making (68). In a randomized control trial Delgado et al. utilized the Go Wish card Game (GWG) and found that patients with advanced cancer gave high importance to having their family present with them, second after spirituality (69). Caregiver symptom burden and perception of their loved ones’ depression (70) may affect their ability to provide adequate care to the patient, which is crucial for care of patient at home. Multiple tools have been developed to capture different aspects of care-giver distress (71). Unfortunately, most of these questionnaires are lengthy, time consuming and used for research purposes. Performing a care-giver ESAS is a quick and feasible tool tested by Tanco et al. (72).
Family education regarding the signs near the time of death
Providing timely education to family regarding the physical signs near time of death can help with addressing questions related to prognostication. Most of these signs could also be seen in severe shock like conditions, however these are not unambiguous. Appropriate and timely delivery of education as patient nears the end of life is of paramount significance to assist the family thru the coping process (73,74) (Table 2).
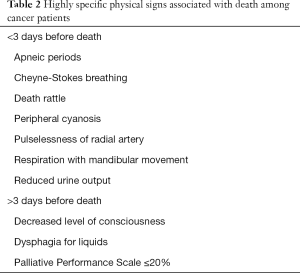
Full table
Family meetings, goals of care conferences
Goals of care conference among medical teams and family care-givers, with or without the patient, can be effective tools in addressing family distress and providing timely education regarding the process of death and expectations of the family members and care-givers (75). However, there is limited understanding about the proper method of conducting such conferences and their outcomes (76).
Despite inter-personal variability in symptom ratings among different professionals, patients and care-givers (10,11,31,34), family members can identify areas of potential intervention for symptom relief (10). Early involvement of family in the care also provides opportunities for education of family members regarding signs and symptoms along the disease trajectory (10). In situation when patients become delirious and/or are unable to complete assessments particularly near the time of death, the family members’ accounts of complaints and assessment ratings may provide as an aide for feasible and timely interventions for symptom relief (9,10).
Understanding the structure and components of the family as a unit is a major element of quality palliative care provision. It includes an overall holistic interdisciplinary approach which provides psychosocial support for the whole family as a unit, centered round the patient, not just during their chronic life-limiting illness but also addresses grief and bereavement after the death of a loved one (51,76,77) focusing on preventing complicated and pathological grief among the bereaved (78-80).
History of substance use
Social assessment includes issues like substance use, which can be measured using tools like the Cut down, Annoyed, Guilty, Eye-opener (CAGE)-adapted to include drugs (CAGE-AID) (81-84) and Screener and Opioid Assessment for Patient’s with Pain-Revised (SOAPP-R; revised version with 14 items) (85-89) questionnaires. CAGE questionnaire is 4 item validated tool to screen for history of alcoholism. A score ≥1 in females and ≥2 in males is considered positive with 85% sensitivity and 90% specificity to detect alcohol abuse and/or dependency. Previous studies have found that patients who have a positive history of smoking, positive CAGE or high score on the SOAPP questionnaires, (SOAPP-R, being the revised shorter version with 14 questions), present with high pain symptom expression, show non-compliance with opioid use recommendations and exhibit aberrant drug behavior (90-94).
These assessments are easier carried out early on in the trajectory of illness. Detection of such predictors can assist clinicians in identifying such behaviors earlier, potentially providing opportunities for proactive interventions, like involvement of Interdisciplinary approaches including compassionate high alert team (CHAT) and performing random urinary drug testing (95-98) in out-patient clinic to improve outcomes.
Death, a spiritual experience!
Spiritual pain is common (more than 40%) among advanced cancer patients (67) and is noted to be associated with lower self-perceived religiosity and spiritual QoL (99). Palliative care teams need to be aware of the strong spiritual and religious needs of the patients with life-threatening illnesses (100,101). In a randomized control trial Delgado et al. utilized the GWG and found that patients with advanced cancer gave high importance to spirituality (to be at peace with God and to pray) (69). Spirituality, religiosity, and spiritual pain may affect symptom expression and coping skills of patients with advanced illnesses. Addressing these concerns is paramount to enhancing the QoL of such patients.
ESAS-Spiritual pain is one of the two new variables (along with financial distress) added to the ESAS and measured in a similar way like other ESAS dimensions on the 11-point scale from 0 to 10, and can be used as a quick tool for assessment. It is well correlated with physical and psychological distress (64,67).
Care-givers who report greater spiritual pain, also report worse psychological distress and worse QoL (102), supporting the importance of spiritual assessment of caregivers (72).
Faith, Importance and Influence, Community, and Address (FICA) Spiritual History Tool (101,103,104) is also a feasible tool for clinical assessment of spirituality.
Functional Assessment of Chronic Illness Therapy--Spiritual Well-Being (FACIT-Sp) is another tool used in surveys (101) and in randomized controlled trials, supporting early integration of palliative care for advanced cancer patients (2).
Some of the other tools developed globally for measurement of PROs include:
- Palliative Care outcome Scale (POS) (6,105,106) is a family of outcome measurement tools that provides opportunity to measure the physical, emotional as well as spiritual and social needs of patients, over a wide spectrum of illnesses including cancer and non-cancerous conditions both in-patient and out-patient (107). These are validated for use in different clinical settings as well as research and available in different languages. POS is delivered as patient, care-giver and staff questionnaires, each consisting of 12 questions;
- POS Symptom list (POS-S) (106) comprises ten symptoms and two open questions. It can be used alongside POS to capture more information about symptoms, and is especially useful where a patient has multiple symptoms. It assesses patient physical symptoms on 5-scale numerical rating system from 0 to 4 (“not at all” to “over-whelming”). For majority of patients near the end of life a pure numerical rated scale may not be feasible to complete and simpler options for rating symptoms like “not at all”, “slightly”, “overwhelmingly and so on can ease communication;
- African version of the POS (106,108) has been validated in resource-poor settings. It consists of seven patient-related and three family care-giver questions rated on a 6-point scale, from 0 to 5;
- Integrated Palliative care Outcome Scale (IPOS) is the integrated version of the above versions (105,106,108,109), which is a short instrument for holistic assessment and has also been validated in a range of settings. It is considered a more streamlined measure which is brief, yet still captures the most important concerns—both in relation to symptoms, but also extending to information needs, practical concerns, anxiety or low mood, family anxieties, and overall feeling of being at peace;
- Other QoL questionnaires include the FACIT group of questionnaires which also aid in assessment of different dimensions. Palliative care subscale (FACIT-Pal) is a lengthy 46-item questionnaire assessing physical, social/family, emotional and functional well-being, on a 5-point rating scale from 0 to 4 (“not at all” to “very much”), over the last 7-day period (110). FACIT-Pal 14 is a shortened version (111,112). FACIT--Spiritual Well-Being (FACIT-Sp) is another tool used in surveys (101) and in randomized controlled trials supporting early integration of palliative care for advanced cancer patients (2);
- European Organization for Research and Treatment of Cancer (EORTC) QoL questionnaires (QLQ) provide range of options for assessment in different dimensions, with EORTC-QLQ-C15-PAL being the shortened version of EORTC-QLC-C30 (112-116), consisting of 14 questions assessing physical well-being and functional domains on a 4-point rating scale 1 to 4 (“not at all” to “very much”) with question 15 assessing overall QoL on a Likert scale 1 (very poor) to 7 (excellent);
- Symptom Assessment Scale (SAS) is an Australian validated numeric rating symptom score where 0= “no distress” experienced from the problem and 10= the “worst imaginable distress experienced”. Symptoms covered by the SAS include bowel problems, pain, difficulty sleeping, nausea, breathing problems, appetite problems, and fatigue. It mainly serves as a screening tool to summarize the amount of distress from a symptom that in turn dictates the urgency of conducting a comprehensive assessment (117,118).
These QoL questionnaires are ideally self-reported but when not possible, the family or, in the absence of, nursing staff (proxy) may score these tools in some cases.
Time is of the essence… which tools to use and how often?
With so many outcome measurement tools available, all these tools are not intended for use in all or even most case. Care teams may select the tools most suitable to the needs of their patient population and practice patterns. The most basic assessments that can be performed in more general settings can be carried out by using tools like ESAS, for most common PROs including the physical and emotional domains, POS-S or SAS. In addition, screening for delirium can be performed using very simple tools like CAM and screening for opioid-misuse risk, when opioids are being considered, by using the CAGE questionnaires.
For patients who eventually get referred to specialized teams of palliative care professionals, the assessments are usually more in-depth and may use tools like revised ESAS (ESAS-FS) to assess additional domains of distress related to financial issues and spiritual distress, more sophisticated assessments for opioid misuse screening like SOAPP-R, more sophisticated instruments for assessments of delirium like MDAS and frequently more in-depth assessments of family and care-giver network.
Which of these evaluations should be conducted every single encounter or repeated periodically as compared to just as a onetime assessment? For some elements like the CAGE and smoking history, it might not be necessary to do more than once if it is done properly. Assessments like ESAS (18,19), which takes a short time to be completed (22), can be self-completed by the patient or family member or assisted by a nurse at the bedside and provides comprehensive information about not only the physical but also the psychological, emotional, social and spiritual issues, as well as POS-S, IPOS (106) and SAS (118) which are also quick and provide a global, multi-dimensional assessment of PROs, it may be appropriate to perform periodically at each patient encounter. For other tools it is not clear how often those should be repeated. Further research is needed to understand the feasibility of such assessments to develop global tools which are simple and easy to use especially for patients near the end of life.
There is no gold standard tool for assessment of different symptoms that patient with advanced illnesses especially near the time of death experience. Many of the available tools measure dimensions which are similar and decision to use a particular tool over the other lies with team of palliative care providers based on practice patterns, geographical location and experiences. While some of these tools are developed and used routinely for clinical and practical implementation, others are used for research purposes (14). There is need for more global multi-center collaboration to understand these complex interactions (119). Table 3 lists some commonly available instruments which can be used both in out-patient supportive and palliative care clinic and in-patient to quickly measure patient report outcomes.
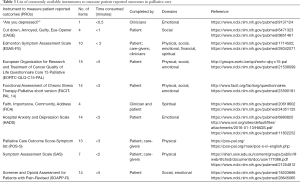
Full table
Conclusions
A global, multi-dimensional assessment of PROs is paramount to providing a holistic multi-disciplinary quality palliative care experience for these patients. While patients may be suffering from physical symptoms of pain, nausea and fatigue due to burden of the illness and associated treatments, they may be facing social isolation and lack of family support (e.g., divorce) while having financial toxicity and suffering from spiritual pain (Figure 1) (1). In addition, evaluation of care-giver burden should be performed to provide needed support for a positive coping experience and to prevent complicated grief. At the same time daily bedside clinical practice requires that we conduct the evaluation of the patients in a timely and effective manner without imposing on the patients, their families and also the healthcare delivery system. Successful implementation and utilization of PRO measures may be better achieved by not only presence of the palliative care clinicians, to provide relief from physical symptoms, but also involvement of a multi-disciplinary team including counselor, palliative care psychologist, chaplain and social worker to address the multiple other dimensions that contribute to overall suffering for the patients and care-givers (109,120).
Acknowledgements
We appreciate Mrs. Elida G. Galan, Senior Administrative Assistant, Department of Palliative Care, Rehabilitation and Integrative Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA, for her support in formatting the figure and tables.
Footnote
Conflicts of Interest: Dr. E Bruera has received research funding grant from Helsinn Pharmaceuticals. A Azhar has no conflicts of interest to declare.
References
- Dalal S, Bruera E. End-of-Life Care Matters: Palliative Cancer Care Results in Better Care and Lower Costs. Oncologist 2017;22:361-8. [Crossref] [PubMed]
- Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. [Crossref] [PubMed]
- Grossman SA. Undertreatment of cancer pain: barriers and remedies. Support Care Cancer 1993;1:74-8. [Crossref] [PubMed]
- Potter VT, Wiseman CE, Dunn SM, et al. Patient barriers to optimal cancer pain control. Psychooncology 2003;12:153-60. [Crossref] [PubMed]
- Etkind SN, Daveson BA, Kwok W, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015;49:611-24. [Crossref] [PubMed]
- Guo P, Gao W, Higginson IJ, et al. Implementing Outcome Measures in Palliative Care. J Palliat Med 2018;21:414. [Crossref] [PubMed]
- Tran K, Zomer S, Chadder J, et al. Measuring patient-reported outcomes to improve cancer care in Canada: an analysis of provincial survey data. Curr Oncol 2018;25:176-9. [Crossref] [PubMed]
- Hosie A, Davidson PM, Agar M, et al. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med 2013;27:486-98. [Crossref] [PubMed]
- Kristjanson LJ, Nikoletti S, Porock D, et al. Congruence between patients' and family caregivers' perceptions of symptom distress in patients with terminal cancer. J Palliat Care 1998;14:24-32. [PubMed]
- Nekolaichuk CL, Maguire TO, Suarez-Almazor M, et al. Assessing the reliability of patient, nurse, and family caregiver symptom ratings in hospitalized advanced cancer patients. J Clin Oncol 1999;17:3621-30. [Crossref] [PubMed]
- Nekolaichuk CL, Bruera E, Spachynski K, et al. A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med 1999;13:311-23. [Crossref] [PubMed]
- ACGME. ACGME Program Requirements for Graduate Medical Education in Hospice and Palliative Medicine 2018. Accessed August 3rd, 2018. Available online: https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/540HospicePalliativeMedicine1YR2018.pdf?ver=2018-02-16-083923-063
- The Joint Commission Certification for Palliative Care Programs 2017. Accessed May 31st, 2018. Available online: https://www.jointcommission.org/certification/palliative_care.aspx
- van Roij J, Fransen H, van de Poll-Franse L, et al. Measuring health-related quality of life in patients with advanced cancer: a systematic review of self-administered measurement instruments. Qual Life Res 2018;27:1937-1955. [Crossref] [PubMed]
- Ledoux M, Rhondali W, Lafumas V, et al. Palliative care referral and associated outcomes among patients with cancer in the last 2 weeks of life. BMJ Support Palliat Care 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Hui D, Meng YC, Bruera S, et al. Referral Criteria for Outpatient Palliative Cancer Care: A Systematic Review. Oncologist 2016;21:895-901. [Crossref] [PubMed]
- Hui D, Mori M, Watanabe SM, et al. Referral criteria for outpatient specialty palliative cancer care: an international consensus. Lancet Oncol 2016;17:e552-9. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J Pain Symptom Manage 2017;53:630-43. [Crossref] [PubMed]
- Chasen M, Bhargava R, Dalzell C, et al. Attitudes of oncologists towards palliative care and the Edmonton Symptom Assessment System (ESAS) at an Ontario cancer center in Canada. Support Care Cancer 2015;23:769-78. [Crossref] [PubMed]
- Pereira JL, Chasen MR, Molloy S, et al. Cancer Care Professionals' Attitudes Toward Systematic Standardized Symptom Assessment and the Edmonton Symptom Assessment System After Large-Scale Population-Based Implementation in Ontario, Canada. J Pain Symptom Manage 2016;51:662-72.e8. [Crossref] [PubMed]
- Wong A, Rodriguez-Nunez A, Tayjasanant S, et al. Edmonton Symptom Assessment Scale (ESAS): Time duration of self-completion versus assisted-completion in palliative care patients—A randomized controlled trial. J Clin Oncol 2016;34:67.
- Hui D, Shamieh O, Paiva CE, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015;121:3027-35. [Crossref] [PubMed]
- Hui D, Shamieh O, Paiva CE, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage 2016;51:262-9. [Crossref] [PubMed]
- Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer 1990;65:1864-6. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002-7. [Crossref] [PubMed]
- Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187-93. [Crossref] [PubMed]
- Anderson F, Downing GM, Hill J, et al. Palliative performance scale (PPS): a new tool. J Palliat Care 1996;12:5-11. [PubMed]
- Olajide O, Hanson L, Usher BM, et al. Validation of the palliative performance scale in the acute tertiary care hospital setting. J Palliat Med 2007;10:111-7. [Crossref] [PubMed]
- Kim YJ, Hui D, Zhang Y, et al. Differences in Performance Status Assessment Among Palliative Care Specialists, Nurses, and Medical Oncologists. J Pain Symptom Manage 2015;49:1050-8.e2. [Crossref] [PubMed]
- Chow R, Chiu N, Bruera E, et al. Inter-rater reliability in performance status assessment among health care professionals: a systematic review. Ann Palliat Med 2016;5:83-92. [Crossref] [PubMed]
- Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer 2010;46:3175-83. [Crossref] [PubMed]
- Zimmermann C, Burman D, Bandukwala S, et al. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support Care Cancer 2010;18:609-16. [Crossref] [PubMed]
- de Kock I, Mirhosseini M, Lau F, et al. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care 2013;29:163-9. [PubMed]
- Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med 2012;15:902-9. [Crossref] [PubMed]
- Hui D, dos Santos R, Reddy S, et al. Acute symptomatic complications among patients with advanced cancer admitted to acute palliative care units: A prospective observational study. Palliat Med 2015;29:826-33. [Crossref] [PubMed]
- Breitbart W, Alici Y. Agitation and delirium at the end of life: "We couldn't manage him". JAMA 2008;300:2898-910, E1.
- Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183-94. [Crossref] [PubMed]
- Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer 2009;115:2004-12. [Crossref] [PubMed]
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. [Crossref] [PubMed]
- Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128-37. [Crossref] [PubMed]
- Lawlor PG, Nekolaichuk C, Gagnon B, et al. Clinical utility, factor analysis, and further validation of the memorial delirium assessment scale in patients with advanced cancer: Assessing delirium in advanced cancer. Cancer 2000;88:2859-67. [Crossref] [PubMed]
- Bush SH, Bruera E. The assessment and management of delirium in cancer patients. Oncologist 2009;14:1039-49. [Crossref] [PubMed]
- Bush SH, Kanji S, Pereira JL, et al. Treating an established episode of delirium in palliative care: expert opinion and review of the current evidence base with recommendations for future development. J Pain Symptom Manage 2014;48:231-48. [Crossref] [PubMed]
- Daeninck PJ, Bruera E. Opioid use in cancer pain. Is a more liberal approach enhancing toxicity? Acta Anaesthesiol Scand 1999;43:924-38. [Crossref] [PubMed]
- Gaudreau JD, Gagnon P, Harel F, et al. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol 2005;23:6712-8. [Crossref] [PubMed]
- Jackson N, Doherty J, Coulter S. Neuropsychiatric complications of commonly used palliative care drugs. Postgrad Med J 2008;84:121-6. [Crossref] [PubMed]
- Morita T, Tei Y, Tsunoda J, et al. Underlying pathologies and their associations with clinical features in terminal delirium of cancer patients. J Pain Symptom Manage 2001;22:997-1006. [Crossref] [PubMed]
- Lapane KL, Hughes CM, Daiello LA, et al. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc 2011;59:1238-45. [Crossref] [PubMed]
- Bruera E, Higginson I, Von Gunten CF, et al. Textbook of palliative medicine. 2nd edition. Boca Raton: CRC Press/Taylor & Francis Group, 2015. xxxiii, 1322 pages.
- Yennurajalingam S, Bruera E. Oxford American handbook of hospice and palliative medicine and supportive care. 2nd edition. New York: Oxford University Press, 2016. xv, 510 pages.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. [Crossref] [PubMed]
- Fan SY, Lin WC, Lin IM. Psychosocial care and the role of clinical psychologists in palliative care. Am J Hosp Palliat Care 2015;32:861-8. [Crossref] [PubMed]
- Chochinov HM, Wilson KG, Enns M, et al. "Are you depressed?" Screening for depression in the terminally ill. Am J Psychiatry 1997;154:674-6. [Crossref] [PubMed]
- Killgore WD. The visual analogue mood scale: can a single-item scale accurately classify depressive mood state? Psychol Rep 1999;85:1238-43. [Crossref] [PubMed]
- Beck A, Steer R. Beck depression inventory manual. San Antonio: Psychological Corporation, 1987.
- Chibnall JT, Tait RC. The short form of the Beck Depression Inventory: validity issues with chronic pain patients. Clin J Pain 1994;10:261-6. [Crossref] [PubMed]
- Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord 2005;86:87-91. [Crossref] [PubMed]
- Chochinov HM, McClement S, Hack T, et al. Eliciting Personhood Within Clinical Practice: Effects on Patients, Families, and Health Care Providers. J Pain Symptom Manage 2015;49:974-80.e2. [Crossref] [PubMed]
- Pan JL, Chochinov H, Thompson G, et al. The TIME Questionnaire: A tool for eliciting personhood and enhancing dignity in nursing homes. Geriatr Nurs 2016;37:273-7. [Crossref] [PubMed]
- Azhar A, Yennurajalingam S, Ramu A, et al. Timing of Referral and Characteristics of Uninsured, Medicaid, and Insured Patients Referred to the Outpatient Supportive Care Center at a Comprehensive Cancer Center. J Pain Symptom Manage 2018;55:973-8. [Crossref] [PubMed]
- Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial Distress and Its Associations With Physical and Emotional Symptoms and Quality of Life Among Advanced Cancer Patients. Oncologist 2015;20:1092-8. [Crossref] [PubMed]
- de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017;123:476-84. [Crossref] [PubMed]
- de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer 2014;120:3245-53. [Crossref] [PubMed]
- Delgado-Guay MO, Chisholm G, Williams J, et al. Frequency, intensity, and correlates of spiritual pain in advanced cancer patients assessed in a supportive/palliative care clinic. Palliat Support Care 2016;14:341-8. [Crossref] [PubMed]
- Yennurajalingam S, Parsons HA, Duarte ER, et al. Decisional control preferences of Hispanic patients with advanced cancer from the United States and Latin America. J Pain Symptom Manage 2013;46:376-85. [Crossref] [PubMed]
- Delgado-Guay MO, Rodriguez-Nunez A, De la Cruz V, et al. Advanced cancer patients' reported wishes at the end of life: a randomized controlled trial. Support Care Cancer 2016;24:4273-81. [Crossref] [PubMed]
- Rhondali W, Chirac A, Laurent A, et al. Family caregivers' perceptions of depression in patients with advanced cancer: a qualitative study. Palliat Support Care 2015;13:443-50. [Crossref] [PubMed]
- Tanco K, Park JC, Cerana A, et al. A systematic review of instruments assessing dimensions of distress among caregivers of adult and pediatric cancer patients. Palliat Support Care 2017;15:110-24. [Crossref] [PubMed]
- Tanco K, Vidal M, Arthur J, et al. Testing the feasibility of using the Edmonton Symptom Assessment System (ESAS) to assess caregiver symptom burden. Palliat Support Care 2018;16:14-22. [Crossref] [PubMed]
- Hui D, dos Santos R, Chisholm G, et al. Clinical signs of impending death in cancer patients. Oncologist 2014;19:681-7. [Crossref] [PubMed]
- Hui D, Dos Santos R, Chisholm G, et al. Bedside clinical signs associated with impending death in patients with advanced cancer: preliminary findings of a prospective, longitudinal cohort study. Cancer 2015;121:960-7. [Crossref] [PubMed]
- Rhondali W, Dev R, Barbaret C, et al. Family conferences in palliative care: a survey of health care providers in France. J Pain Symptom Manage 2014;48:1117-24. [Crossref] [PubMed]
- Dev R, Coulson L, Del Fabbro E, et al. A prospective study of family conferences: effects of patient presence on emotional expression and end-of-life discussions. J Pain Symptom Manage 2013;46:536-45. [Crossref] [PubMed]
- Del Ferraro C, Ferrell B, Van Zyl C, et al. Improving Palliative Cancer Care. J Adv Pract Oncol 2014;5:331-8. [Crossref] [PubMed]
- Ghesquiere A, Haidar YM, Shear MK. Risks for complicated grief in family caregivers. J Soc Work End Life Palliat Care 2011;7:216-40. [Crossref] [PubMed]
- Shear MK. Clinical practice. Complicated grief. N Engl J Med 2015;372:153-60. [Crossref] [PubMed]
- Shear MK, Ghesquiere A, Glickman K. Bereavement and complicated grief. Curr Psychiatry Rep 2013;15:406. [Crossref] [PubMed]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252:1905-7. [Crossref] [PubMed]
- Ewing JA. Screening for alcoholism using CAGE. Cut down, Annoyed, Guilty, Eye opener. JAMA 1998;280:1904-5. [Crossref] [PubMed]
- Parsons HA, Delgado-Guay MO, El Osta B, et al. Alcoholism screening in patients with advanced cancer: impact on symptom burden and opioid use. J Palliat Med 2008;11:964-8. [Crossref] [PubMed]
- Couwenbergh C, Van Der Gaag RJ, Koeter M, et al. Screening for substance abuse among adolescents validity of the CAGE-AID in youth mental health care. Subst Use Misuse 2009;44:823-34. [Crossref] [PubMed]
- Koyyalagunta D, Bruera E, Engle MP, et al. Compliance with Opioid Therapy: Distinguishing Clinical Characteristics and Demographics Among Patients with Cancer Pain. Pain Med 2018;19:1469-77. [Crossref] [PubMed]
- Akbik H, Butler SF, Budman SH, et al. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP). J Pain Symptom Manage 2006;32:287-93. [Crossref] [PubMed]
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain 2008;9:360-72. [Crossref] [PubMed]
- Dale R, Edwards J, Ballantyne J. Opioid risk assessment in palliative medicine. J Community Support Oncol 2016;14:94-100. [Crossref] [PubMed]
- Finkelman MD, Jamison RN, Kulich RJ, et al. Cross-validation of short forms of the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R). Drug Alcohol Depend 2017;178:94-100. [Crossref] [PubMed]
- Kim YJ, Dev R, Reddy A, et al. Association Between Tobacco Use, Symptom Expression, and Alcohol and Illicit Drug Use in Advanced Cancer Patients. J Pain Symptom Manage 2016;51:762-8. [Crossref] [PubMed]
- Childers JW, King LA, Arnold RM. Chronic Pain and Risk Factors for Opioid Misuse in a Palliative Care Clinic. Am J Hosp Palliat Care 2015;32:654-9. [Crossref] [PubMed]
- Dev R, Parsons HA, Palla S, et al. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer 2011;117:4551-6. [Crossref] [PubMed]
- Kwon JH, Hui D, Chisholm G, et al. Predictors of long-term opioid treatment among patients who receive chemoradiation for head and neck cancer. Oncologist 2013;18:768-74. [Crossref] [PubMed]
- Kwon JH, Tanco K, Park JC, et al. Frequency, Predictors, and Medical Record Documentation of Chemical Coping Among Advanced Cancer Patients. Oncologist 2015;20:692-7. [Crossref] [PubMed]
- Arthur J, Edwards T, Reddy S, et al. Outcomes of a Specialized Interdisciplinary Approach for Patients with Cancer with Aberrant Opioid-Related Behavior. Oncologist 2018;23:263-70. [Crossref] [PubMed]
- Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 2016;122:3732-9. [Crossref] [PubMed]
- Arthur JA, Haider A, Edwards T, et al. Aberrant Opioid Use and Urine Drug Testing in Outpatient Palliative Care. J Palliat Med 2016;19:778-82. [Crossref] [PubMed]
- Pimentel LE, De La Cruz M, Wong A, et al. Snapshot of an Outpatient Supportive Care Center at a Comprehensive Cancer Center. J Palliat Med 2017;20:433-6. [Crossref] [PubMed]
- Delgado-Guay MO, Hui D, Parsons HA, et al. Spirituality, religiosity, and spiritual pain in advanced cancer patients. J Pain Symptom Manage 2011;41:986-94. [Crossref] [PubMed]
- Delgado-Guay MO. Spirituality and religiosity in supportive and palliative care. Curr Opin Support Palliat Care 2014;8:308-13. [Crossref] [PubMed]
- Hills J, Paice JA, Cameron JR, et al. Spirituality and distress in palliative care consultation. J Palliat Med 2005;8:782-8. [Crossref] [PubMed]
- Delgado-Guay MO, Parsons HA, Hui D, et al. Spirituality, religiosity, and spiritual pain among caregivers of patients with advanced cancer. Am J Hosp Palliat Care 2013;30:455-61. [Crossref] [PubMed]
- Borneman T, Ferrell B, Puchalski CM. Evaluation of the FICA Tool for Spiritual Assessment. J Pain Symptom Manage 2010;40:163-73. [Crossref] [PubMed]
- Puchalski CM. The FICA Spiritual History Tool #274. J Palliat Med 2014;17:105-6. [Crossref] [PubMed]
- Collins ES, Witt J, Bausewein C, et al. A Systematic Review of the Use of the Palliative Care Outcome Scale and the Support Team Assessment Schedule in Palliative Care. J Pain Symptom Manage 2015;50:842-53.e19. [Crossref] [PubMed]
- Higginson DIJ. Palliative Care Outcome Scale (POS) 1999. Accessed May 24th, 2018. Available online: https://pos-pal.org
- Stevens AM, Gwilliam B, A'Hern R, et al. Experience in the use of the palliative care outcome scale. Support Care Cancer 2005;13:1027-34. [Crossref] [PubMed]
- Dix O. Impact of the APCA African Palliative Outcome Scale (POS) on care and practice. 2012. Available online: https://www.kcl.ac.uk/nursing/departments/cicelysaunders/attachments/Impact-of-the-APCA-African-Palliative-care-Outcome-Scale-(POS)-on-care-and-practice.pdf
- Antunes B, Harding R, Higginson IJ, et al. Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med 2014;28:158-75. [Crossref] [PubMed]
- Lyons KD, Bakitas M, Hegel MT, et al. Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative care (FACIT-Pal) scale. J Pain Symptom Manage 2009;37:23-32. [Crossref] [PubMed]
- Zeng L, Bedard G, Cella D, et al. Preliminary results of the generation of a shortened quality-of-life assessment for patients with advanced cancer: the FACIT-Pal-14. J Palliat Med 2013;16:509-15. [Crossref] [PubMed]
- Chiu L, Chiu N, Chow E, et al. Comparison of three shortened questionnaires for assessment of quality of life in advanced cancer. J Palliat Med 2014;17:918-23. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55-64. [Crossref] [PubMed]
- Ganesh V, Zhang L, Wan BA, et al. Symptom clusters using the EORTC QLQ-C15-PAL in palliative radiotherapy. Ann Palliat Med 2018;7:192-204. [Crossref] [PubMed]
- McDonald R, Ding K, Chow E, et al. Classification of painful bone metastases as mild, moderate, or severe using both EORTC QLQ-C15-PAL and EORTC QLQ-BM22. Support Care Cancer 2016;24:4871-8. [Crossref] [PubMed]
- Snyder CF, Blackford AL, Sussman J, et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients' supportive care needs. Qual Life Res 2015;24:1207-16. [Crossref] [PubMed]
- Clark K, Connolly A, Clapham S, et al. Physical Symptoms at the Time of Dying Was Diagnosed: A Consecutive Cohort Study to Describe the Prevalence and Intensity of Problems Experienced by Imminently Dying Palliative Care Patients by Diagnosis and Place of Care. J Palliat Med 2016;19:1288-95. [Crossref] [PubMed]
- Aoun SM, Monterosso L, Kristjanson LJ, et al. Measuring symptom distress in palliative care: psychometric properties of the Symptom Assessment Scale (SAS). J Palliat Med 2011;14:315-21. [Crossref] [PubMed]
- Kamal AH, Bull J, Kavalieratos D, et al. Development of the Quality Data Collection Tool for Prospective Quality Assessment and Reporting in Palliative Care. J Palliat Med 2016;19:1148-55. [Crossref] [PubMed]
- Tavares AP, Paparelli C, Kishimoto CS, et al. Implementing a patient-centred outcome measure in daily routine in a specialist palliative care inpatient hospital unit: An observational study. Palliat Med 2017;31:275-82. [Crossref] [PubMed]


