Predictors of dyspnea in patients with advanced cancer
Introduction
Dyspnea is one of the most common and debilitating symptoms among advanced cancer patients, affecting up to 70% of patients (1,2). This symptom is characterized by breathlessness and discomfort while breathing (3). The mechanism of dyspnea is not well understood due to the heterogeneous origins of shortness of breath sensations including the brain stem, upper airways, lungs and chest wall and the multitude of causal agents that can contribute to sensations of dyspnea (3). Dyspnea is a significant predictor of poor disease progression, validated by a literature review of 38 studies conducted by the European Association for Palliative Care which found a definitive link between dyspnea and poor prognosis (4). Dyspnea also negatively affects quality of life (QOL) in cancer patients and has been shown through multiple studies to interfere with daily activities and overall enjoyment of life (5-7).
Several assessments have been used to document the incidence and severity of dyspnea, including the Edmonton Symptom Assessment System (ESAS). This 9-item symptom screen measures pain, tiredness, drowsiness, nausea, lack of appetite, shortness of breath, depression, anxiety, and wellbeing on a scale from 0 to 10 based on the patients’ condition at the time of completion. This assessment, combined with the Patient-Reported Functional Status (PRFS), has been validated and used frequently for patients with advanced cancer to assist in the identification of cancer-related symptoms (8-10).
Although the literature surrounding dyspnea has been increasing, there is still need for a greater understanding of this deleterious cancer symptom and the factors that lead to its presentation. This study sought to explore possible predictors of dyspnea among advanced cancer patients in a palliative outpatient radiotherapy clinic.
Methods
A prospective database was collected and reviewed. The study was approved by the hospital research ethics board (No. 391-2016). Sunnybrook research ethics board had determined that an Informed Consent Form was not required for this study.
Patient population
All patients attending the Rapid Response Radiotherapy Program (RRRP) at the OCC in Toronto, Canada from February 2016 to April 2017 were eligible for inclusion in the present study. The RRRP is a palliative outpatient radiotherapy clinic that delivers radiation treatment to advanced cancer patients with the aim of alleviating symptoms from painful bone metastases to improve their QOL.
Data collection
All patients attending the RRRP completed a combined ESAS and PRFS assessment prior to seeing the radiation oncologist in clinic. Afterwards, a clinical research assistant measured their oxygen saturation using a pulse oximeter and collected information on smoking history, history of respiratory conditions, primary cancer site and other patient and disease characteristics. Some patients had undergone multiple courses of radiotherapy, usually in the form of a re-treatment for refractory pain. If patients attended the clinic multiple times, their information was collected at these visits as well.
ESAS severity
The shortness of breath item on the ESAS was used to characterize the severity of dyspnea as none (0), mild [1–3], moderate [4–7], and severe [8–10].
Statistical analysis
Although some patients attended the clinic multiple times within the study period, demographics were only conducted for the patients’ first visit. Very few patients had 5 or more visits; therefore, these results were not included in the analysis. Demographics were summarized using median age in years, and proportions for categorical variables.
To search for associations between dyspnea severity (none, mild, moderate, and severe) and other predictive factors, univariate ordinal logistic regression analysis was used to create a cumulative logit prediction model using dyspnea severity and collected factors as possible predictors. In the multivariable analysis, the stepwise (backward) selection procedure of ordinal logistic regression was conducted using potential variables with P<0.10 obtained from univariate analysis. The final model only included the significant predictors after adjusting for other covariates. R2 was also applied for measure of fit in the modeling (11). R2 indicates the proportion of variability in the dyspnea score which can be explained by the predictive factors (12,13).
With patients that had multiple visits, dyspnea severity was measured repeatedly over time and the generalized estimating equation (GEE) method was used to fit models for correlated data arising from repeated measurements (11,13). The ordinal outcome was repeated measurements of dyspnea severity and the independent variables were time and each demographic factor. Time was calculated in months since the first measurement. The quasi-likelihood information criterion (QIC) was estimated for model goodness of fit criteria (14). All significant variables (P<0.10) from the univariate GEE analysis were included in the multivariable analysis to search for the variables most significantly related to dyspnea severity after accounting for time.
The above analysis was repeated to search for the association of moderate/severe dyspnea (ESAS score ≥4) with other predictive factors, at the patient’s first visit and over time.
All calculations were performed using Statistical Analysis Software (SAS version 9.4 for Windows). SAS Logistic and GENMOD Procedures were applied for statistical modeling. P values <0.05 were considered statistically significant.
Results
Demographics
A summary of patient characteristics is displayed in Table 1. A total of 252 patients were included in the study; the median age was 71.3 years and most participants were male (n=155, 61.5%). The most common cancer sites included prostate (n=60, 23.8%), lung (n=56, 22.2%) and gastrointestinal (n=34, 13.5%). The most common sites of metastases were bone (n=209, 82.9%), liver (n=68, 27.0%), lymph node (n=67, 26.6%) and lung (n=61, 24.2%). An overview of the number of patients with multiple visits can be found in Table 2. At the first visit, 51 (20.2%), 36 (14.3%), and 25 (9.9%) patients had mild, moderate, and severe dyspnea, respectively. Overall, dyspnea was present in 112 patients (44.4%) and moderate/severe dyspnea was present in 61 (24.2%) of patients. 101 patients (40.1%) had a PRFS ≥3, indicative of weakness and poor QOL. Presence of moderate/severe dyspnea was highest in lung (n=20, 35.7%) and prostate cancer patients (n=12, 20.0%).
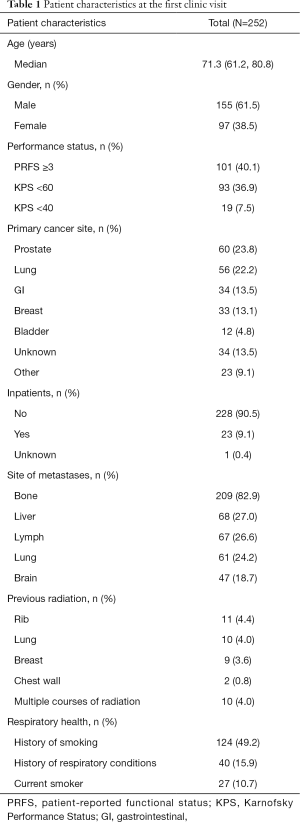
Full table
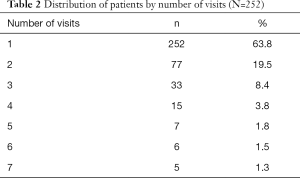
Full table
Predictors of dyspnea severity
In univariate analysis, liver metastases, PRFS ≥3, pulse oximetry <90 and history of respiratory conditions were significant predictors of a greater severity of dyspnea (Table 3). In the multivariable analysis, patients with liver metastases, a history of respiratory conditions, or PRFS ≥3 were more likely to have a greater severity of dyspnea symptoms, compared to other factors (Table 3).
After adjusting for time in the univariate analysis, patients with liver or lymph node metastases, KPS <40, PRFS ≥3, pulse oximetry <90, or a history of respiratory conditions were more likely to have a greater severity of dyspnea symptoms (Table 3). Patients with previous multiple courses of radiation treatment were less likely to have severe dyspnea symptoms than other factors. For the multivariable analysis, after accounting for the non-significant time effect, patients with lymph node or liver metastases, pulse oximetry <90 or a history of respiratory conditions were predictive of greater dyspnea severity (Table 3). Patients with previous multiple courses of radiation were less likely to have severe dyspnea symptoms than other variables. The distribution of dyspnea severity in patients did not significantly change over time.
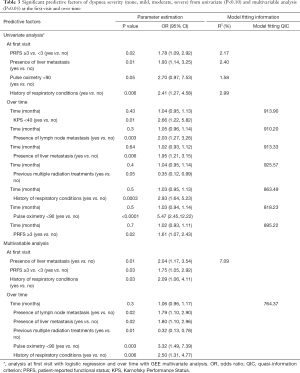
Full table
Predictors of moderate/severe dyspnea
At the first visit, univariate logistic regression analysis showed patients with lung metastases, a history of respiratory conditions or PRFS ≥3 were more likely to have moderate/severe dyspnea symptoms (score ≥4), compared to other factors (Table 4). In the multivariable analysis, these predictive factors remained significant (Table 4).
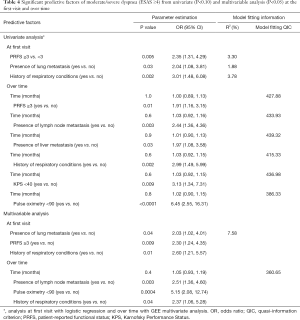
Full table
From univariate GEE analysis over time, patients with liver or lymph node metastases, KPS <40, pulse oximetry <90, PRFS ≥3 or a history of respiratory conditions were more likely to have moderate/severe dyspnea (Table 4). After accounting for the non-significant time effect in the multivariable analysis, patients with lymph node metastases, pulse oximetry <90, or a history of respiratory conditions were more likely to have moderate/severe dyspnea (Table 4). The proportion of moderate/severe dyspnea within the patient population did not significantly change over time.
Secondary analysis of the role of liver and lymph metastases
A secondary analysis of these results was performed to better characterize the relationship between lymph and liver metastases and functional status (PRFS ≥3, KPS <40 and KPS <60) in order to determine a clinical correlation for the significant association of lymph and liver metastases with dyspnea. Spearman correlation coefficients (r) between lymph metastases and PRFS ≥3 displayed significance (r=0.15; P=0.02), although no significant correlations between liver metastases and KPS/PRFS were observed. For dyspnea severity and presence of moderate/severe dyspnea, non-significant PRFS ≥3, KPS <40 and/or KPS <60 variables from the univariate analysis were placed into the final multivariable models as potential confounding factors where liver and lymph metastases were found to be significant. Liver and lymph metastases remained significant after accounting for KPS/PRFS in all analyses. Also, in the univariate logistic regression analysis for the presence of liver metastases, no significant relationships were found between liver metastases and PRFS ≥3, KPS <40 or KPS <60. However, in the subsequent analysis for the presence of lymph metastases, PRFS ≥3 displayed significance (P=0.03), indicating that those with a lower functional status are more likely to have lymph metastases.
Discussion
In this study, after multivariable analysis, liver and lung metastases, PRFS ≥3 and a history of respiratory conditions were predictive of dyspnea symptoms, measured as the severity of dyspnea or the presence of moderate/severe dyspnea at the first visit. Over time, liver and lymph node metastases, a history of respiratory conditions, PRFS ≥3 and pulse oximetry <90 were predictive of dyspnea symptoms. Patients with multiple radiation treatments were less likely to have severe dyspnea symptoms at the first visit and over time.
A history of respiratory conditions as a significant predictor of dyspnea has been validated in the literature through multiple studies such as Dudgeon et al.’s prospective survey study (n=923) that reported a significant correlation of dyspnea with previous chronic obstructive pulmonary disease (COPD) (P=0.019) or asthma (P=0.001) (15). A review of the pathophysiology of dyspnea by Manning et al. also noted similar mechanisms of action between common respiratory conditions and dyspnea (3). Interestingly, in the present study, lung metastases were predictive of moderate/severe dyspnea at the first visit but did not display statistical significance for the severity of dyspnea and did not show significance for either of these categories over time. A primary lung cancer diagnosis also did not display significance for these categories even though the presence of moderate/severe dyspnea was highest in lung cancer patients (n=20, 35.7%). A prospective study by Bruera et al. (n=135) found that lung involvement, either by primary tumour or metastases, was associated with the intensity of dyspnea, but was not an independent correlate of moderate/severe dyspnea, indicating that lung involvement is not a consistent predictor of dyspnea severity (2). Additionally, a study by Reuben et al. (n=1,754) found that 75% of patients with primary or metastatic lung involvement had previously reported dyspnea, but these patients constituted only 39% of patients reporting shortness of breath (1). This shows that dyspnea is a multidimensional symptom and although lung involvement may be correlated with dyspnea, it is not a consistent predictor.
In this analysis, PRFS ≥3 was predictive of increased severity of dyspnea symptoms and greater presence of moderate/severe dyspnea at the first visit. This could be explained by a multitude of studies that show that the frequency and severity of dyspnea is dependent on the stage of disease and worsens at the end of life (16-19). PRFS levels 3 and 4 are indicative of little ability to perform activities and most time spent sitting or lying down, which are characteristics often observed at the end of life when dyspnea symptoms are most apparent. Also, liver and lymph node metastases were predictive of the severity of dyspnea in various analyses. These findings are validated in the literature as lymph node metastases are indicative of the spread of cancer to other regions of the body via the lymphatic system and have been found to be a potential prognostic indicator in affected patients (20). Similarly, liver metastases have been correlated with poor patient outcomes and worse overall survival compared to other metastatic sites in pancreatic and prostate cancer patients (21,22). These findings in the literature as well as the results of our secondary analysis investigating the role of lymph and liver metastases show the association of liver and lymph metastases and limited functional status with advanced disease progression, providing a plausible mechanism for their significant association with dyspnea in our analyses as dyspnea worsens with disease progression. However, due to the conflicting results in our secondary analysis, more research should be done to confirm the correlation of these symptoms with dyspnea and their relationship with disease progression.
Karnofsky Performance Status (KPS) <40 and <60 in this analysis did not reach significance. A study by Mercadante et al. found that the intensity of dyspnea began to increase around KPS 60, reaching its peak intensity and frequency at levels KPS 30 and KPS 20 (16). Our findings are inconsistent with the literature, where it has been demonstrated that low KPS grades are significantly correlated with dyspnea (1). The Karnofsky Performance Status was measured by a clinical research assistant or health care provider during the patient’s visit, presupposing a possibility that based on the short appointment time and subjective opinion of the recorder, a full depiction of the patient’s functional status was not accurately assessed. Furthermore, due to the outpatient nature of the clinic where this data was collected, patients require a certain degree of mobility to attend, providing an explanation for the study population consisting of only 36.9% (n=93) and 7.5% (n=19) patients with KPS 60 and KPS 40, respectively. The limited number of patients that displayed these characteristics could have potentially interfered with the results.
Multivariate analysis found patients with multiple courses of radiation treatment were less likely to have severe dyspnea symptoms over time. These results could be attributed to the therapeutic action of radiation, although it should be noted that most patients with multiple treatments were analyzed for symptoms within a month of one or both of their radiation treatments. Since clinical benefits from radiation can take between days to several months to manifest and since there was a small number of patients (n=10, 4.0%) with multiple treatments, these results may be misleading.
Since the sample size for patients with 2, 3 or 4 visits (n=77; n=33; n=15) was much fewer than the records available at the first visit (n=252), the accuracy of the GEE analysis over time may have been compromised. Certain predictors only reached significance over multiple visits including lymph node metastases and pulse oximetry <90. Few to no studies have reported on these measures, therefore, with the absence of available correlation with the literature, these results should be considered with caution.
The nature of the ESAS presents some difficulty regarding the accurate characterization of dyspnea symptoms as the survey only collects data at the time the patient fills out the assessment. A study by Reddy et al. found that continuous dyspnea was less prevalent than breakthrough dyspnea, raising the concern that though the patient was not experiencing dyspnea at the time of ESAS completion, they could have experienced episodes of dyspnea recently (6). The inability of the ESAS to accurately distinguish continuous and breakthrough dyspnea reflects a significant limitation of this study.
This study could be improved with the addition of cardiac related patient outcomes within the univariate and multivariable analyses as cardiac complications have been reported to be associated with dyspnea severity (1,23). Also, the addition of pulmonary function measures such as maximal inspiratory pressure, vital capacity and forced expiratory volume have been investigated in the literature and further research could validate previously studied predictors of dyspnea (2,23-25). Furthermore, this study’s conclusions with regard to the relationship between lymph metastases and dyspnea may have been limited by the inclusion of all lymphatic regions within the definition of lymph metastases. Since lung involvement has been significantly associated with dyspnea, lymph metastases found only in the mediastinal region would perhaps have been a better predictor of the presence and severity of dyspnea than lymph metastases anywhere in the body.
The identification of predictive factors for dyspnea could help health care providers to recognize dyspnea earlier and develop better treatments for this distressing symptom. One such model that has been proposed is a rapid learning health care model that features continuous generation and analysis of patient data to ensure constant quality improvement and acknowledgement of the various modalities through which dyspnea can present, allowing for a fuller understanding of patient experience (26). As more predictive factors are identified, a more comprehensive understanding of the psychosocial and clinical indicators of dyspnea can be obtained, leading to more effective treatments for patients.
Also, although several screening tools exist, none consider all the factors that can affect dyspnea presence and severity. The most routinely used scale to measure dyspnea is the Dyspnea Visual Analogue Scale (DVAS) and less frequently used scales include the Dyspnea Verbal Rating Scale (DVRS), Dyspnea Interview Schedule, Chronic Respiratory Questionnaire, Pulmonary Functional Status Scale, Baseline Dyspnea Index and more (27). Several studies have found a significant correlation when comparing the VAS, VRS and numeric rating scales, such as the ESAS, for the characterization of pain (28,29). However, due to the distinct properties of these screening tools and the interpretability of dyspnea based on subjective experience, equivalent comparisons and substitutability between screening tools in the literature may be difficult to demonstrate. Although the use of the ESAS in this study was sufficient to characterize the presence and severity of dyspnea, more detailed questionnaires utilized in conjunction or succession with this survey could better distinguish an individual patient’s experience with dyspnea. These tools are used in research but there is little literature documenting the frequency with which these are used in clinical practice to identify dyspnea symptoms in patients. As further evidence is published confirming and identifying dyspnea predictors, comprehensive screening tools can be created to allow for early detection of dyspnea symptoms in a clinical setting. Care should be taken to ensure that screening tools are comprehensive but not excessive in length as assessments that are overly difficult to complete may detract from the QOL of advanced cancer patients.
Conclusions
The present study identifies predictors of dyspnea as liver, lung and lymph node metastases, a history of respiratory conditions, PRFS ≥3 and pulse oximetry <90. These results show dyspnea to be a multidimensional symptom that is perhaps better characterized as an indicator of overall staging of disease progression rather than a complication of lung-related conditions. These results can help health care providers assess the risk of dyspnea in late stage cancer patients and allow for the treatment of dyspnea with a holistic approach, addressing diverse factors that could lead to dyspnea symptoms.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund. We thank Medigas for the use of the pulse oximeter device.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the hospital research ethics board (No. 391-2016). Sunnybrook research ethics board determined that an Informed Consent Form was not required for this study.
References
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest 1986;89:234-6. [Crossref] [PubMed]
- Bruera E, Schmitz B, Pither J, et al. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage 2000;19:357-62. [Crossref] [PubMed]
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 1995;333:1547-53. [Crossref] [PubMed]
- Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations: a study by the steering committee of the European Association for Palliative Care. J Clin Oncol 2005;23:6240-8. [Crossref] [PubMed]
- Gupta D, Lis CG, Grutsch JF. The relationship between dyspnea and patient satisfaction with quality of life in advanced cancer. Support Care Cancer 2007;15:533-8. [Crossref] [PubMed]
- Reddy SK, Parsons HA, Elsayem A, et al. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med 2009;12:29-36. [Crossref] [PubMed]
- Tanaka K, Akechi T, Okuyama T, et al. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manage 2002;23:417-23. [Crossref] [PubMed]
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164-71. [Crossref] [PubMed]
- Yogananda MN, Muthu V, Prasad KT, et al. Utility of the revised Edmonton Symptom Assessment System (ESAS-r) and the Patient-Reported Functional Status (PRFS) in lung cancer patients. Support Care Cancer 2018;26:767-75. [Crossref] [PubMed]
- Bruera E, Neumann C, Brenneis C, et al. Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care 2000;16:16-21. [PubMed]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13-22. [Crossref]
- SAS Insitute. SAS/STAT 9.4 User’s Guide: The Logistic Procedure. Cary, NC.
- Nelder JA, Wedderburn RWM. Generalized Linear Models. J R Stat Soc Ser A 1972;135:370. [Crossref]
- Pan W. Akaike’s Information Criterion in Generalized Estimating Equations. Biometrics 2001;57:120-5. [Crossref] [PubMed]
- Dudgeon DJ, Kristjanson L, Sloan JA, et al. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage 2001;21:95-102. [Crossref] [PubMed]
- Mercadante S, Casuccio A, Fulfaro F. The course of symptom frequency and intensity in advanced cancer patients followed at home. J Pain Symptom Manage 2000;20:104-12. [Crossref] [PubMed]
- Hardy JR, Turner R, Saunders M, et al. Prediction of survival in a hospital-based continuing care unit. Eur J Cancer 1994;30A:284-8. [Crossref] [PubMed]
- Escalante CP, Martin CG, Elting LS, et al. Dyspnea in cancer patients: etiology, resource utilization, and survival-implications in a managed care world. Cancer 1996;78:1314-9. [Crossref] [PubMed]
- Teunissen SC, de Graeff A, de Haes HC, et al. Prognostic significance of symptoms of hospitalised advanced cancer patients. Eur J Cancer 2006;42:2510-6. [Crossref] [PubMed]
- Carr I. Lymphatic metastasis. Cancer Metastasis Rev 1983;2:307-17. [Crossref] [PubMed]
- Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol 2017;23:1872-80. [Crossref] [PubMed]
- Shou J, Zhang Q, Wang S, et al. The prognosis of different distant metastases pattern in prostate cancer: a population based retrospective study. Prostate 2018;78:491-7. [Crossref] [PubMed]
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage 1998;16:212-9. [Crossref] [PubMed]
- Heyse-Moore L, Beynon T, Ross V. Does spirometry predict dyspnoea in advanced cancer? Palliat Med 2000;14:189-95. [Crossref] [PubMed]
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage 2001;21:373-9. [Crossref] [PubMed]
- Abernethy AP, Kamal AH, Wheeler JL, et al. Management of dyspnea within a rapid learning healthcare model. Curr Opin Support Palliat Care 2011;5:101-10. [Crossref] [PubMed]
- Mancini I, Body JJ. Assessment of dyspnea in advanced cancer patients. Support Care Cancer 1999;7:229-32. [Crossref] [PubMed]
- Averbuch M, Katzper M. Assessment of visual analog versus categorical scale for measurement of osteoarthritis pain. J Clin Pharmacol 2004;44:368-72. [Crossref] [PubMed]
- Jones KR, Vojir CP, Hutt E, et al. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev 2007;44:305-14. [Crossref] [PubMed]


