Evaluation of the 3-day recall period for the Functional Life Index-Emesis (FLIE)
Introduction
Nausea and vomiting are common side effects of many cancer treatments, and can have significant impacts on patient quality of life (QoL) (1,2). These distressing symptoms are often experienced by patients receiving radiation therapy (3,4). Radiation-induced nausea and vomiting (RINV) has been reported with rates as high as 80% in patients receiving abdominal radiation; overall, rates of RINV range from 30–40% (2,4).
Because QoL is often adversely affected by RINV, several recent trials employed by our centre testing the efficacy of various anti-emetic medications have included a QoL tool as a standard questionnaire (5,6). To assess the QoL of patients experiencing RINV, the Functional Life Index-Emesis (FLIE) was used for these studies. The FLIE is a QoL questionnaire that evaluates the prevalence of nausea and vomiting and their effect on different aspects of QoL and function (7). The FLIE uses a 7-point scale ranging from 1 (not at all) to 7 (a great deal) for its 18 questions, with Q1–9 assessing presence of nausea and its effects on various life aspects, and Q10–18 assessing that of vomiting. This measurement tool employs a recall period of 3 days (7).
The use of recall periods by questionnaires has been debated in the past. One study by Lackner et al. showed that the accuracy of retrospective symptom questionnaires is not well-understood. When asked to report on their experiences over the past 7 days, patients with gastrointestinal symptoms were fairly accurate in recalling certain experiences, such as the most severe pain that occurred, but tended to overestimate other measures, such as the average intensity of pain (8). Norquist et al. remarked in their review of patient-reported outcome measures employing recall periods that the use and length of recall periods on such questionnaires must depend on what exactly is being measured, the nature of symptoms, the impact of patient burden and the feasibility of recalling details of the symptoms in question (9). Depending on the nature of the symptoms being measured, different recall periods may be indicated for an accurate representation of the patient’s experience; for example, frequently measuring symptoms that tend to be consistent over time would pose an unnecessary burden on patients, and infrequently measuring symptoms that occur very sporadically would likely be ineffective in capturing their impact on the patient (9).
As the FLIE used in the current evaluated studies of antiemetic medications employs a recall period, our objective was to demonstrate its effectiveness in reporting QoL symptoms accurately, or its lack thereof (5,6,10).
Methods
This was a secondary analysis of data on nausea and vomiting collected in three separate trials which enrolled palliative oncology patients at the Odette Cancer Centre. Patients received medium or low emetogenic risk palliative radiotherapy of either a single 8 Gy fraction, 20 Gy in 5 fractions, or 30 Gy in 10 fractions according to the 2009 Multinational Association for Supportive Care in Cancer (MASCC) guidelines, which were standard at time of study enrollment (11). Radiotherapy to upper abdomen or upper half body radiation were considered to have moderate emetogenic risk, and radiotherapy to cranium, craniospinal, head and neck, lower thorax, and pelvis were considered to have low emetogenic risk (2). The three trials that contributed data to this study were:
A phase II pilot study on ondansetron rapidly dissolving film (Ondissolve) for the prophylactic treatment of RINV (6).
A pilot study investigating the efficacy of aprepitant and granisetron for the prophylaxis of RINV (5).
A prospective study of palonosetron in RINV (10).
All individuals enrolled had a Karnofsky performance status of 40 or greater. The studies were approved by the Hospital Research Ethics Board and Health Canada (ondansetron: No. 102-2013, aprepitant/granisetron: No. 257-2010; palonosetron: No. 434-2013), and patients provided written informed consent prior to enrollment.
For the duration of radiotherapy treatment and for 10 days after radiotherapy completion, patients completed diaries in which they recorded their daily severity of nausea and of vomiting as “none”, “mild”, “moderate”, or “severe”.
For the ondansetron study, patients completed the FLIE at baseline, and at days 3 and 7 during treatment. In the aprepitant/granisetron study, patients completed the FLIE at baseline and at days 5 and 10 of the follow-up period. For the Palonosetron study, patients completed the FLIE on days 5 and 10 of treatment if they received multiple fraction radiation, and days 3 and 7 follow-up. Each administration of the FLIE and the three daily diaries associated with the recall period (the day of and two days prior to the day of FLIE completion) were considered a “block”. Treatment or follow-up days 1–3 were considered block A, days 3–5 were block B, days 5–7 were block C, and days 7–10 were block D. For each block, the average of the three daily diary scores were calculated.
Statistical analysis
Available patient demographic data were summarised using descriptive statistics. Responses for patients who have non-zero scores for the FLIE Q1 (how much nausea have you had in the past 3 days?) or Q10 (how much vomiting have you had in the past 3 days?), and non-zero scores for the associated daily general nausea and vomiting questions were analysed. For example, analysis for FLIE completed at follow-up day 10 utilized the nausea and vomiting scores on diary days 8, 9, and 10 follow-ups. If an individual completed more than one FLIE, a random block was selected for analysis such that those who completed multiple FLIE questionnaires were not overrepresented.
Descriptive statistical analyses were performed to summarise demographic information, using mean, standard deviation (SD), median, inter-quartiles, and range for continuous variables, and percentages for categorical variables. To calculate concordance between the FLIE nausea question (Q1) and the daily, most severe, and three-day average responses to nausea severity, scores from both scales were first transformed to a continuous scale of 0–100, where “0” represented no nausea, and “100” represented a FLIE nausea score of “7” or a daily diary score of “severe”. The concordance correlation coefficient (rc) with associated 95% confidence interval (CI) was then calculated. A value of rc =+1 corresponds to perfect agreement; a value of rc =−1 corresponds to perfect negative agreement; and a value of rc =0 corresponds to no agreement. The same procedure was performed for vomiting, using FLIE vomiting question (Q10) and the daily responses to vomiting severity. Similarly, the concordance correlation coefficient was calculated between the FLIE nausea or vomiting question and the daily, most severe, or 3-day average response of “enjoyment of life”. Statistical analysis was performed using Statistical Analysis Software (SAS version 9.4 for Windows, Cary, NC, USA).
Results
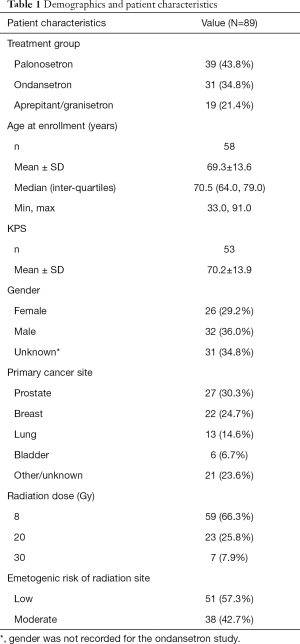
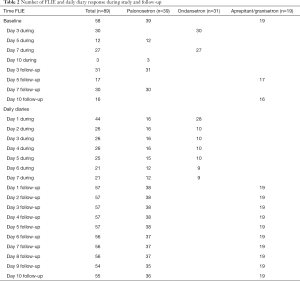
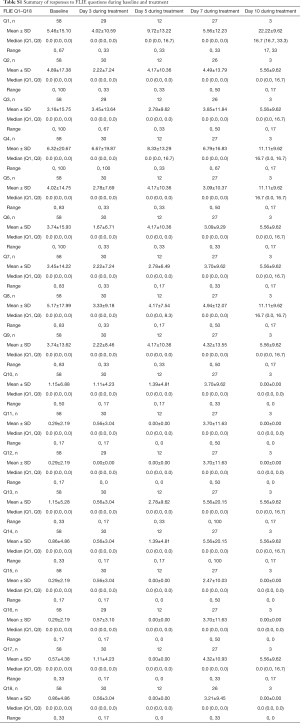
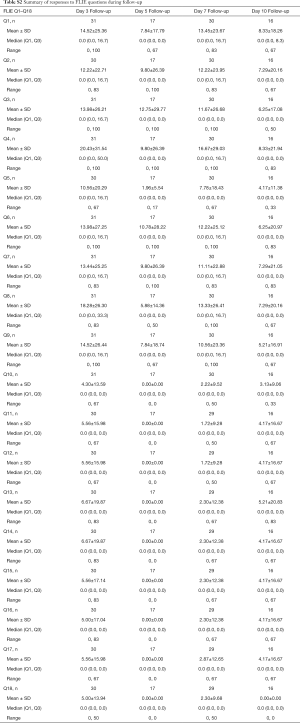
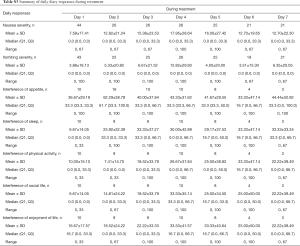
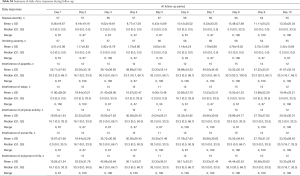
Patient demographics are presented on Table 1. In total, there were 89 patients who had nausea or vomiting who were analysed, of which 39 were from the palonosetron study, 31 were from the ondansetron study, and 19 were from the aprepitant/granisetron study. Table 2 summarises the number of patients who had FLIE responses with nausea or vomiting according to the three anti-emetic trials. Summaries of the scaled responses to FLIE questions 1–18 for all studies are presented on Table S1 for responses at baseline and during treatment, and on Table S2 for responses during follow-up. Summaries of the scaled responses to the daily diary questions on nausea and vomiting are presented on Table S3 for during treatment, and Table S4 for during follow-up.
Full table
Full table
Full table
Full table
Full table
Full table
As Table 3 shows, the highest overall concordance for FLIE nausea Q1 was found to be with the 3-day average of the daily diary nausea responses for both the treatment period (rc =0.698, 95% CI: 0.495, 0.829) and the follow-up period (rc =0.821, 95% CI: 0.711, 0.892). On the other hand, the FLIE vomiting Q10 had the highest concordance with the daily diary vomiting responses on the worst day during treatment (rc =0.310, 95% CI: 0.194, 0.417) or the two days prior during follow-up periods (rc =0.902, 95% CI: 0.832, 0.944).

Full table
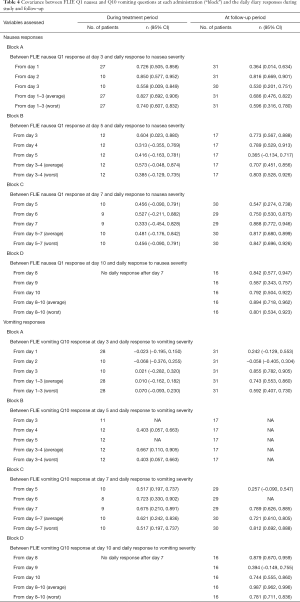
The concordance between FLIE nausea Q1 and the daily responses to nausea severity according to each block are presented on Table 4; concordances for vomiting are on Table 4. The day with the highest concordance appeared to vary with the date of FLIE administration. The strongest rc for FLIE administered during the treatment period was found for early on in the treatment period at Block A, where FLIE responses at day 3 were most closely correlated with the 1-day prior (day 2) response to nausea (rc =0.850, 95% CI: 0.577, 0.952). At the follow-up period, the strongest concordance was between FLIE administered on day 10 follow-up and the average of the three-day diary responses (rc =0.894, 95% CI: 0.718, 0.962). For FLIE vomiting scores during treatment, the strongest concordance was found between the FLIE vomiting response at day 7 and the 1-day prior (day 6) daily diary response (rc =0.723, 95% CI: 0.330, 0.902). At follow-up, the strongest concordance was between the FLIE administered on day 10 of follow-up and the 3-day average daily diary response (rc =0.987, 95% CI: 0.962, 0.996).
Full table
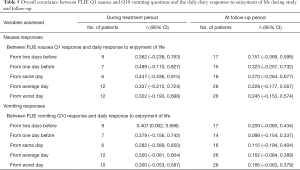
To determine which day the FLIE nausea and vomiting responses best reflected enjoyment of life, the calculated concordances were compared and presented on Table 5. FLIE nausea responses had the highest concordance with the one-day prior enjoyment of life response during treatment (rc=0.489, 95% CI: −0.110, 0.827) and during follow-up (rc =0.323, 95% CI: −0.257, 0.723). For the FLIE vomiting response, the highest concordance was with the enjoyment of life question answered two-days before during treatment (rc =0.407, 95% CI: 0.002, 0.698) and follow-up (rc =0.200, 95% CI: −0.060, 0.434).
Full table
Table 6 presents the results concordance between FLIE nausea and vomiting with daily diary responses for enjoyment of life within individual blocks. The highest concordance for nausea and QoL during treatment was found in block B for the prior day response (rc =0.696, 95% CI: −0.933, 0.998). For follow-up, the highest concordance was in block D, for the 3-day average response (rc =0.817, 95% CI: 0.241, 0.967). For vomiting responses, the highest concordance during treatment was found in Block C, corresponding to the prior day daily diary response (rc =0.429, 95% CI: −0.999, 0.999). During follow-up, highest concordance was on prior day response and FLIE vomiting in block D (rc =0.458, 95% CI: −0.999, 0.999).

Full table
Discussion
Overall, the results suggested that FLIE questionnaires are most representative of the average experience of nausea across the three-day recall period. However, due to low sample sizes, especially during treatment period, the rc values were associated with very large CIs in many blocks. Results for the FLIE vomiting question suggested that it is slightly more representative of the vomiting experienced on the worst day, or the first day within the three-day recall period. However, in both cases for vomiting, the concordance of the average day response followed very closely. When concordance was evaluated within each administration of FLIE (within a block), the differences between the daily diary response became more pronounced, but also more inconsistent with regards to whether the highest concordance was with daily responses from a particular day, the worst day, or average of the 3 days. Therefore, this variation likely contributed to an averaging of the individual effects, which led to a reduced overall concordance correlation.
Analyses of cancer pain reporting have suggested that recall of worst pain, rather than pain averaged across a recall period, better reflects the overall experience of pain (12). Our results have found that the worst nausea or vomiting does correlate adequately with FLIE nausea and vomiting, but it is not consistently the response with the best concordance ratio when compared with responses from other days or the average of the three days.
Analysis of FLIE nausea and vomiting with enjoyment of life showed that there was a weak and inconsistent relationship with the particular date of enjoyment of life assessed. This variation pointed to the difficulty of assessing and interpreting the results from different symptom measures as even within a single tool, there was significant variation depending on the particular association investigated and period of administration.
One limitation of our study was the small sample size, which limited the generalisability and the clinical significance of the findings. This was particularly problematic for the vomiting scores and the enjoyment of life analysis. Very few patients experienced vomiting and we therefore had low numbers eligible for analysis. Larger studies are needed for smaller CIs and for more definitive interpretation of the results.
This study contributes to an area of study with limited existing research. The determination of whether the FLIE represents average, daily, or most severe nausea and vomiting will allow healthcare providers to better interpret patient-reported symptoms, and will inform the design and wording of surveys to more accurately reflect the aspect of the symptom they are aiming to evaluate.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Hospital Research Ethics Board and Health Canada (ondansetron: No. 102-2013, aprepitant/granisetron: No. 257-2010; palonosetron: No. 434-2013) and patients provided written informed consent prior to enrollment.
References
- Hesketh PJ. Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2008;358:2482-94. [Crossref] [PubMed]
- Enblom A, Bergius Axelsson B, Steineck G, et al. One third of patients with radiotherapy-induced nausea consider their antiemetic treatment insufficient. Support Care Cancer 2009;17:23-32. [Crossref] [PubMed]
- Dennis K, Jamani R, McGrath C, et al. A systematic review of methodologies, endpoints, and outcome measures in randomized trials of radiation therapy-induced nausea and vomiting. Support Care Cancer 2017;25:2019-33. [Crossref] [PubMed]
- Maranzano E, De Angelis V, Pergolizzi S, et al. A prospective observational trial on emesis in radiotherapy: Analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol 2010;94:36-41. [Crossref] [PubMed]
- Dennis K, De Angelis C, Jon F, et al. Aprepitant and granisetron for the prophylaxis of radiotherapy-induced nausea and vomiting after moderately emetogenic radiotherapy for bone metastases: A prospective pilot study. Curr Oncol 2014;21:e760-7. [Crossref] [PubMed]
- Wong E, Pulenzas N, Bedard G, et al. Ondansetron rapidly dissolving film for the prophylactic treatment of radiation-induced nausea and vomiting—a pilot study. Curr Oncol 2015;22:199-210. [Crossref] [PubMed]
- Lindley CM, Hirsch JD, O’Neill CV, et al. Quality of life consequences of emesis. Qual Life Res 1992;1:331-40. [Crossref] [PubMed]
- Lackner JM, Jaccard J, Keefer L, et al. The accuracy of patient-reported measures for GI symptoms: A comparison of real time and retrospective reports. Neurogastroenterol Motil 2014;26:1802-11. [Crossref] [PubMed]
- Norquist JM, Girman C, Fehnel S, et al. Choice of recall period for patient-reported outcome (PRO) measures: Criteria for consideration. Qual Life Res 2012;21:1013-20. [Crossref] [PubMed]
- ClinicalTrials.gov. Prospective Study of Palonosetron in Radiation Induced Nausea and Vomiting (RINV) [Internet]. Identifier NCT02388750. Cited 2017 Nov 28. Available online: https://clinicaltrials.gov/ct2/show/NCT02388750
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol 2010;21:v232-43. [Crossref] [PubMed]
- Shi Q, Wang XS, Mendoza TR, et al. Assessing persistent cancer pain: a comparison of current pain ratings and pain recalled from the past week. J Pain Symptom Manage 2009;37:168-74. [PubMed]


