Prevalence and intensity of dyspnea, pain, and agitation among people dying with late stage dementia compared with people dying with advanced cancer: a single-center preliminary study in Brazil
Introduction
Palliative care aims to provide the best care possible for patients facing life-threatening illness and support for their families (1). Although cancer remains the most common condition among patients referred to specialized palliative care programs (2,3), the importance to extend palliative care services to people with non-malignant diseases such as dementia has been widely recognized (4).
In most cases, dementia is a progressive and incurable neurodegenerative disorder, and the average survival time after dementia diagnosis is around 8 years (5). Around the world, several palliative care programs are now serving people with dementia (6). In a recent study we found that advanced cancer and late stage dementia were the two most common underlying diagnosis among people admitted to a palliative care program in Brazil (7). Unfortunately, the organization and quality of palliative care services are usually better for cancer than for dementia (8). In fact, people with dementia are more likely to receive inappropriate end of life care than those who are cognitively intact (9).
Despite the undoubtful benefits of palliative care, the evidence supporting effective strategies for dementia is not as robust as the evidence for cancer. Data from isolated studies suggest that there may be similarities in terms of dyspnea, pain, and agitation among people dying with dementia and people dying with cancer (10-13). However, little is known about how dementia compares with cancer when death approaches. Because transition from cure to palliation often occurs late in the dying process, such comparative analysis would be useful to improve the quality of protocols on the domain of physical comfort, and to guide the provision of palliative care services across different patient populations and care settings. Therefore, the purpose of this study was to estimate the prevalence and intensity of dyspnea, pain, and agitation among people dying with late stage dementia compared with those dying with advanced cancer.
Methods
Study setting and the palliative care program
Located in Rio de Janeiro, Brazil, The Hospital Placi is a private, 30-bed post-acute care facility (PACF). In Brazil, PACFs usually provide multiple services (skilled nursing facility, inpatient functional rehabilitation, and hospice care) at the same location. A palliative care program has been fully integrated into the main portfolio of post-acute care services. The interdisciplinary palliative care team holds responsibility for documentation of advance care planning, symptom management, psychosocial support, and bereavement interventions.
Symptom assessment
Symptom assessments were performed every day at 10:00 and 20:00, by registered nurses, who were trained to document the Edmonton Symptom Assessment System (ESAS), in a face-to-face visit. The ESAS is a numeric rating scale (NRS) that ranges from 0 (no symptom) to 10 (worst possible), used to quantify nine symptoms (pain, activity, nausea, depression, anxiety, drowsiness, appetite, well-being, dyspnea), and an optional extra symptom (14). Our palliative care team included the symptom “agitation” as the optional extra symptom. The results were systematically documented in the electronic chart. For study purposes, we looked at the last six consecutive ESAS scores of dyspnea, pain, and agitation, from the moment of death backwards in time (3 days). Then, each symptom was categorized as none (ESAS =0), mild (ESAS =1–3), moderate (ESAS =4–7), or severe (ESAS =8–10).
Data collection and criteria
The aim of the study was to estimate the prevalence and intensity of dyspnea, pain, and agitation among dying patients with dementia compared with those dying with cancer. This study used both prospective and retrospective data, which were obtained from electronic health records. We evaluated the hospital database to identify patients with dementia, and patients with cancer, who died from 1 June 2013 to 31 December 2017. Patients were considered eligible for the study if they were at least 18 years old, had a physician’s diagnosis of late stage dementia and a Cognitive Performance Scale of 5 or 6 (15), or advanced cancer (recurrent or metastatic), and had an observation period of at least 3 days before death. If the same patient had dementia, and cancer, the disease was coded as cancer. We also collected general information on admission like age, gender, length of stay (LOS), and baseline Karnofsky Performance Scale (KPS). The ethical committee of the Fluminense Federal University approved the study (No. 64110516.1.0000.5243) and provided waiver of patient consent.
Statistical analysis
We calculated an ideal sample size of 108 patients (54 patients with dementia vs. 54 patients with cancer) to identify a statistical difference of each symptom (dyspnea, pain, and agitation), with a power of 80%. Standard descriptive statistics were used to summarize demographic characteristics. All numeric variables were expressed as mean values, and categorical variables were expressed as absolute numbers, and percentage.
We estimated the prevalence and intensity for each symptom studied, separately for both groups. Symptom intensity was determined as the percentage of patients with symptoms at moderate to severe level (ESAS >4). We used student t-test to compare numeric variables, and Fisher’s exact test or chi-square correlations to evaluate categorical variables. The P value lower than 0.05 was considered significant.
A multivariate logistic regression analysis was also performed, to evaluate independent factors associated with dyspnea, pain, agitation, severe symptoms (ESAS >7), and the co-occurrence of symptoms (at least two symptoms during the last 3 days of life); we performed this analysis with the variables: age, gender, LOS, and the diagnosis of dementia or cancer.
Results
Sample characteristics
Our final study sample comprised 111 deceased patients, 57 patients with dementia, and 54 patients with cancer. Dementia patients were older and had longer LOS compared with cancer patients. We found no differences for gender, and KPS (Table 1).
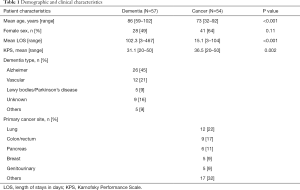
Full table
Prevalence of dyspnea, pain, and agitation: dementia vs. cancer
Dyspnea was detected in 34 (60%) patients with dementia vs. 39 (72%) patients with cancer (P=0.23). Agitation occurred in 7 (13%) patients with dementia vs. 14 (25%) patients with cancer (P=0.17). Pain was identified in 19 (34%) patients with dementia vs. 31 (57%) patients with cancer (P=0.02). These results are summarized in Table 2.

Full table
Symptom intensity: dementia vs. cancer
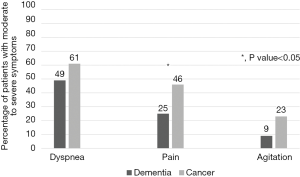
There were no significant differences in the percentage of patients with moderate to severe dyspnea (dementia: n=28, 49% vs. cancer: n=33, 61%; P=0.28), and moderate to severe agitation (dementia: n=4, 7% vs. cancer: n=12, 23%; P=0.09). Patients with dementia were less likely to experience moderate to severe pain (dementia: n=14, 25% vs. cancer: n=25, 46%; P=0.03) than patients with cancer (Figure 1).
Factors associated with dyspnea, pain, agitation, severe symptoms, and the co-occurrence of symptoms
In the multivariate analysis, cancer was the only independent factor associated with pain, [odds ratio (OR): 1.13, P=0.02], along with more severe symptoms (OR: 1.32, P=0.001), and the co-occurrence of symptoms (OR: 1.02, P=0.01).
Discussion
To our best knowledge, this is the first study directly comparing typical end of life symptoms such as dyspnea, pain, and agitation among people dying with dementia and people dying with cancer, using the same tool, and during the same time frame. We limited the analysis to the last 3 days of life in order to better address the period of time where symptoms become more intense, and patients require more palliative care interventions. Our study focused on dyspnea, pain, and agitation because these symptoms have been associated to several negative outcomes at the end of life such as multiple care setting transitions (16), inappropriate use of acute care resources (17), caregivers burden (18), and loss of dignity in terminally ill patients (19).
We found that dyspnea was the most prevalent symptom in both groups of patients. Our findings were in line with studies that showed high prevalence rates of dyspnea among terminally ill patients during their last days of life (10,11,20). In the present study, the vast majority of patients with dementia had had a tube feeding and tracheostomy already in place at the time of palliative care referral. In Brazil, people with late stage dementia are often admitted to intensive care units to receive life sustaining treatments (7). In fact, the use of mechanical ventilation in hospitalized people with dementia has increased over the last decades (21). Because Catholicism strongly prevails in Brazil, many family members do not agree with the decision about withholding or withdrawing hydration and artificial nutrition at the end of life, and requests for interventions that prolong the process of dying are quite common. In this study, delayed palliative care transitions could have contributed to a distinct pattern of symptoms, with less dehydration and cachexia, and more respiratory secretions and dyspnea during the last moments of life (10,22).
Our results showed that approximately one third of patients with dementia, and more than a half of patients with cancer had pain. As expected, people with late stage dementia was not able to give any valid self-reports, so that the level of pain was mainly based on nurse’s judgment or caregiver’s perception. A previous work found that pain assessment in dementia is a controversial issue, with poor correlation between different instruments (23). The use of tools that rely on the observation of pain related behaviors such as facial expressions, body postures, and vocalizations are not accurate, potentially resulting in inappropriate treatment of pain (24). Indeed, cognitive decline is considered an independent risk factor for poor pain control at the end of life (25), and strategies such as enhancing assessment (26) or providing palliative care services may not necessarily improve pain management (27). At the bedside, if pain was suspected, our palliative care team used a pragmatic approach, which consisted of an empiric analgesic trial with opioids, followed by a careful reappraisal of pain related behaviors in a few minutes. Further prospective studies are necessary to identify better tools to manage pain among people with late stage dementia.
Our study provided preliminary information about the prevalence rates of cancer pain in Brazil. Considering that patients were seen by a specialized palliative care team in a private hospital, these rates were probably underestimated, and did not reflect the “real world” in Brazil, where traditionally the access to opioids is limited. Although advances have been made since the World Health Organization meeting of experts aimed at improving cancer pain in Latin America, more than two decades ago (28), gaps in education, social equality, and public policies remain important barriers for effective cancer pain control in Brazil (29).
In this study the rates of agitation were not as impressive as the rates of dyspnea and pain, and lower than other estimates (10,12,13). The reasons behind these results were not well understood. There is a possibility that in the dementia group, the symptom agitation had been overlooked by our palliative care team, and patients erroneously classified as having pain. It is well known that agitation is a multifactorial and complex symptom, often associated with pain in dementia (30). Another plausible explanation is that an intermittent pattern of agitation (sometimes with resolution) can occur among dying patients with dementia (12). In addition, some advanced cancer patients become less agitated, and more somnolent at the end of life (11). Furthermore, opioids, benzodiazepines, and neuroleptics were frequently used in both groups, which could have decreased the overall rates of agitation.
This study has important limitations. First, data on symptom assessment were not collected specifically for research purposes, and the retrospective nature of this study was itself a limitation. Second, we arbitrarily narrowed the list of symptoms to look at dyspnea, pain, and agitation. Other sources of suffering for patients and families were not captured in this study and should be addressed in future research. Third, because of the lack of detailed information on these patients, we could not draw any conclusion about the appropriateness of end of life care delivered. Fourth, we used the ESAS, which is a simple and practical tool, but it has not been validated for dementia. Finally, as the trajectory of functional decline in dementia is usually less abrupt when compared to cancer, people with dementia had longer average LOS than people with cancer. This fact could have resulted in distinct approaches by the palliative care team, between the two groups of patients.
In summary, people dying with late stage dementia experienced rates of dyspnea, and agitation that were similar to people dying with advanced cancer. However, pain was significantly more prevalent and intense among people dying with cancer. Cancer patients were at increased risk for pain, more severe symptoms, and the co-occurrence of dyspnea, pain, and agitation. Given the elevated rates of distressing physical symptoms found in this study, our preliminary findings suggest that there is a wide room for improvement in terms of existing protocols for dyspnea, pain and agitation among people dying with dementia and those dying with cancer in Brazil.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethical committee of the Fluminense Federal University approved the study (No. 64110516.1.0000.5243) and provided waiver of patient consent.
References
- Kelley AS, Morrison RS. Palliative Care for the Seriously Ill. N Engl J Med 2015;373:747-55. [Crossref] [PubMed]
- Ostgathe C, Alt-Epping B, Golla H, et al. Non-cancer patients in specialized palliative care in Germany: What are the problems? Palliat Med 2011;25:148-52. [Crossref] [PubMed]
- West E, Pasman HR, Galesloot C, et al. Hospice Care in Netherlands: who applies and who is admitted to inpatient care? BMC Health Serv Res 2016;16:33. [Crossref] [PubMed]
- van der Steen JT, Radbruch L, Hertogh CM, et al. White paper defining optimal palliative care in older people with dementia: A Delphi study and recommendations from the European Association for Palliative Care. Palliat Med 2014;28:197-209. [Crossref] [PubMed]
- Davies E, Higginson IJ. Better palliative care for older people. World Health Organization Europe 2004.
- Torke AM, Holtz LR, Hui S, et al. Palliative Care for Patients with Dementia: A National Survey. J Am Geriatr Soc 2010;58:2114-21. [Crossref] [PubMed]
- Soares LG, Japiassu AM, Gomes LC, et al. Post-Acute Care Facility as a Discharge Destination for Patients in Need of Palliative Care in Brazil. Am J Hosp Palliat Care 2018;35:198-202. [Crossref] [PubMed]
- Davies N, Maio L, van Riet Paap J, et al. Quality palliative care for cancer and dementia in five European countries: some common challenges. Aging Ment Health 2014;18:400-10. [Crossref] [PubMed]
- Sampson EL, Gould V, Lee D, et al. Differences in care received by patients with and without dementia who died during acute admission: a retrospective case note study. Age Ageing 2006;35:187-9. [Crossref] [PubMed]
- Hendriks SA, Smalbrugge M, Hertogh CM, et al. Dying with dementia: symptoms treatment and quality of life in the last week of life. J Pain Symptom Manage 2014;47:710-20. [Crossref] [PubMed]
- Hui D, dos Santos R, Chisholm GB, et al. Symptom Expression in the Last Seven Days of Life Among Cancer Patients Admitted to Acute Palliative Care Units. J Pain Symptom Manage 2015;50:488-94. [Crossref] [PubMed]
- Hendricks SA, Smalbrugge M, Galindo-Garre F, et al. From Admission to Death: Prevalence and Course of Pain, Agitation, and Shortness of Breath, and Treatment of These Symptoms in Nursing Home Residents with Dementia. J Am Med Dir Assoc 2015;16:475-81. [Crossref] [PubMed]
- Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom Prevalence in Patients with Incurable Cancer: A Systematic Review. J Pain Symptom Manage 2007;34:94-104. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol 1994;49:M174-82. [Crossref] [PubMed]
- Lawson B, Burge FI, Critchley P, et al. Factors associated with multiple transitions in care during the end of life following enrollment in a comprehensive palliative care program. BMC Palliat Care 2006;5:4. [Crossref] [PubMed]
- Grudzen CR, Richardson LD, Morrison M, et al. Palliative Care Needs of Seriously Ill, Older Adults Presenting to the Emergency Department. Acad Emerg Med 2010;17:1253-7. [Crossref] [PubMed]
- Krug K, Miksch A, Peters-Klimm F, et al. Correlation between patient quality of life in palliative care and burden of their family caregivers: a prospective observational cohort study. BMC Palliat Care 2016;15:4. [Crossref] [PubMed]
- Chochinov HM, Hassard T, Kristjanson LJ, et al. Dignity in the terminally Ill: a cross-sectional cohort study. Lancet 2002;360:2026-30. [Crossref] [PubMed]
- Currow DC, Smith J, Davidson PM, et al. Do the trajectories of Dyspnea Differ in Prevalence and Intensity by Diagnosis at the End of Life? A Consecutive Cohort Study. J Pain Symptom Manage 2010;39:680-90. [Crossref] [PubMed]
- Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med 2016;176:1809-16. [Crossref] [PubMed]
- Mitchell SL, Teno JM, Kiely DK, et al. The Clinical Course of Advanced Dementia. N Engl J Med 2009;361:1529-38. [Crossref] [PubMed]
- Closs SJ, Barr B, Briggs M, et al. A Comparison of Five Pain Assessment Scales for Nursing Home Residents with Varying Degrees of Cognitive Impairment. J Pain Symptom Manage 2004;27:196-205. [Crossref] [PubMed]
- Pautex S, Michon A, Guedira M, et al. Pain in Severe Dementia: Self-Assessment or Observational Scales? J Am Geriatr Soc 2006;54:1040-5. [Crossref] [PubMed]
- Klinkenberg M, Willems DL, van de Wal G, et al. Symptom Burden in the Last Week of Life. J Pain Symptom Manage 2004;27:5-13. [Crossref] [PubMed]
- Zwakhalen SM, van’t Hof CE, Hamers JP. Systematic pain assessment using an observational scale in nursing home residents with dementia: exploring feasibility and applied interventions. J Clin Nurs 2012;21:3009-17. [Crossref] [PubMed]
- Kayser Jones JS, Kris AE, Miaskowski CA, et al. Hospice care in nursing homes: does it contribute to higher quality of pain management? Gerontologist 2006;46:325-33. [Crossref] [PubMed]
- Stjernsward J, Bruera E, Joranson D, et al. Opioid availability in Latin America: the declaration of Florianopolis. J Pain Symptom Manage 1995;10:233-6. [Crossref] [PubMed]
- Soares LG. Poor Social Conditions, Criminality and Urban Violence: Unmentioned Barriers for effective cancer pain control at the end of life. J Pain Symptom Manage 2003;26:693-5. [Crossref] [PubMed]
- Sampson EL, White N, Lord K, et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain 2015;156:675-83. [Crossref] [PubMed]


