Management of metastatic spinal cord compression among Veterans Health Administration radiation oncologists
Introduction
The incidence of spinal metastases, with associated complications such as metastatic spinal cord compression (MSCC), is increasing given ongoing improvements in systemic therapy (1). Spinal cord compression has become a common complication that impacts 5% of patients with metastatic disease (2). Symptoms of MSCC include back pain, paresthesias, loss of motor function, and loss of continence. Optimal management of MSCC is important to minimize loss of ambulation, preserve functional status, and maintain quality of life in patients with metastatic disease (1-3). A number of factors can impact treatment results, including pretreatment neurologic function, use of steroids, management with surgery and/or radiotherapy (RT), and timeliness of treatment.
Guidelines have been published by the American College of Radiology (ACR) (4,5) to guide treatment decisions for radiation oncologists (ROs) when managing patients with MSCC. These guidelines provide multidisciplinary expert consensus on use of steroids, surgical decompression, and various RT modalities for MSCC based on the literature. However, to our knowledge, there are no available data regarding how often these guidelines are used or how these guidelines have impacted management of MSCC.
Early diagnosis and treatment of MSCC result in improved functional outcomes (6,7). Early administration of steroids in patients with neurologic deficits is advocated by consensus guidelines (4,5,8) for mitigation of tumor related edema and associated symptoms. There are randomized data demonstrating improved survival and ambulatory function in patients who undergo surgical management followed by RT compared to RT alone (9). Therefore, surgical management is often preferred as initial treatment in patients who are medically fit, especially in the setting of radioresistant tumors. The impact of initial treatment choice on treatment delays is poorly studied. More literature regarding treatment delays in the modern era and whether treatment modality impacts these delays is needed.
Given the need for optimal management of MSCC to maximize quality of life in patients with metastatic disease, especially in light of a rising incidence in spinal bone metastases, studies to further evaluate management of MSCC in the community and identify gaps in knowledge are critical. This survey study was conducted to evaluate management of MSCC among Veterans Health Administration (VHA) ROs, determine familiarity and practice concordance with published ACR guidelines, and evaluate the impact of initial treatment modality on time to treatment.
Methods
Using SurveyMonkey©, a survey consisting of 11 questions and four case scenarios (Table 1) was sent to all 79 VHA ROs at 39 centers. Questions were focused on management strategies for MSCC and evaluated use of steroids, decisions for or against neurosurgery, use of RT, time to treatment, RT fractionation schemes, involvement of palliative care services, and use of re-irradiation. The case scenarios focused on use and timing of RT and/or neurosurgery to determine whether management decisions of VHA ROs are concordant with published ACR guidelines. Information was also collected to determine the percentage of respondents that were board certified, case load of MSCC, and familiarity with the ACR Appropriateness Criteria® Spinal Bone Metastases. Follow-up phone calls were made to encourage completion of the survey. Descriptive statistics and chi square tests were performed to determine whether number of cases seen annually and familiarity with ACR guidelines correlated with responses. Chi-square analysis was also performed to determine whether initial treatment with RT versus neurosurgery impacted time to treatment and whether time to neurosurgical treatment correlated with the presence of on-site neurosurgical services.

Full table
Results
Of the 79 ROs surveyed, 64 responded, yielding an 81.0% response rate. The respondents represent 89.5% of VHA Radiation Oncology Centers. All respondents are board certified (96.9%) or board eligible (3.1%). Of the respondents, 37.5% see 1–5 MSCC cases annually, 37.5% see 6–10 cases, and 25.0% see more than 10 cases of MSCC yearly; 79.4% of those surveyed had read the ACR Appropriateness Criteria® Spinal Bone Metastases.
All respondents reported use of steroid therapy when managing patients with MSCC, with two thirds of respondents administering steroids immediately upon clinical suspicion of spinal cord compression and one third awaiting imaging confirmation prior to administration of steroids.
Of surveyed ROs, 56.5% report on-site neurosurgical services, whereas the remainder refer to off-site neurosurgical teams. When surgical decompression is the recommended initial treatment, 34.5% report neurosurgical intervention within 24 hours of MSCC diagnosis. Neurosurgical intervention occurs within 48 hours for 30.9%, within 72 hours for 21.8%, and in greater than 72 hours for 12.7% of respondents. The presence of on-site neurosurgery did not correlate with timeliness of neurosurgical intervention (χ2=10.102, P=0.12). When RT is the recommended initial treatment of MSCC, it is initiated within 24 hours for 83.9%, within 48 hours for 12.9%, and within 72 hours for 3.2% of respondents. When required, RT is more often initiated within 24 hours as compared to neurosurgery (83.9% vs. 34.5%, P<0.001). The majority (87.3%) of surveyed ROs prefer 30 Gy/10 fractions over shorter or longer fractionation schemes for MSCC in patients with a life expectancy greater than 6 months.
All physicians report use of palliative care services for MSCC, with 52.4% always referring and 47.6% sometimes referring to palliative care. In regards to re-irradiation, 66.1% perform re-irradiation: 30.7% with stereotactic body radiation therapy (SBRT), 17.7% with intensity modulated radiation therapy (IMRT), and 17.7% with conventional RT.
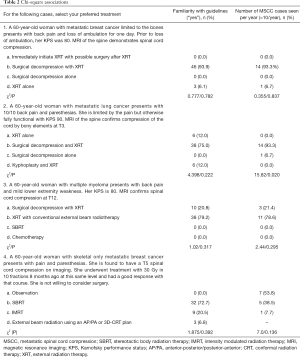
For the clinical case scenarios presented, most respondents’ (>75.0%) management concurred with ACR guidelines. Familiarity with the ACR guidelines or seeing more cases of MSCC per year was not significantly associated with selection of responses concordant with the guidelines (Table 2).
Full table
For the first case scenario, a 60-year-old woman with good performance status and recent loss of ambulation, 93.4% of respondents selected surgical decompression followed by RT, which is the preferred treatment in such a scenario per the ACR guidelines. The remainder (6.6%) selected RT alone.
For the second case scenario, a 60-year-old woman with good performance status and symptoms of back pain and paresthesia, the majority (75.8%) once again selected surgical decompression followed by postoperative RT. Accordingly, surgical decompression followed by RT is the preferred treatment in such a scenario per the ACR guidelines; 11.3% selected RT alone, 11.3% selected kyphoplasty followed by RT, and 1.6% selected surgical decompression alone.
For the third case scenario, a 60-year-old woman with good performance status and mild lower extremity weakness secondary to multiple myeloma, the majority (81.7%) selected conventional RT only, which is appropriate per the ACR guidelines given the radiosensitive nature of multiple myeloma. 18.3% selected surgical decompression followed by RT, and no respondent selected SBRT or chemotherapy alone.
Discussion
Appropriate and timely management of MSCC is essential to optimizing ambulation and functional status in patients with metastatic disease (3). The ACR Appropriateness Criteria® Spinal Bone Metastases (4), and more recently, the publication by Lo et al. (5) provide evidence-based consensus guidelines for the management of MSCC. This survey evaluated the appropriateness, based on ACR guidelines, of MSCC management among VHA ROs. Results of the survey demonstrated general concordance between reported practice among VHA ROs and treatment recommendations in the ACR Appropriateness Criteria® Spinal Bone Metastases. Of note, Lo et al.’s article had not yet been published at the time of this survey.
Steroids are recommended as first-line treatment for spinal cord compression given associated reduction in tumor-related edema and the presence of randomized data demonstrating improved ambulatory function in MSCC patients receiving dexamethasone (2,10). As recommended by the ACR guidelines, all respondents recommended use of steroids for MSCC. Also in concordance with the guidelines, all respondents reported involvement of hospice/palliative care in patients with a life expectancy less than 6 months.
The ACR guidelines recommend consideration for surgical decompression in patients with MSCC and good performance status. Responses to the case scenarios demonstrate that respondents are most likely to recommend surgical decompression in patients with loss of ambulation. In the first case scenario in which a patient with a good performance status had lost ambulation, 93.4% recommended surgical decompression followed by RT. In the second case scenario, which involved a patient with pain and paresthesia secondary to spinal cord compression by bony elements, a lower percentage of 75.8% recommended surgical decompression followed by RT. While the ACR guidelines do not recommend kyphoplasty for management of MSCC, 11.3% recommended kyphoplasty followed by RT for the second case scenario. It is possible that fewer respondents recommended upfront surgery for the second case, compared to the first case, given preservation of motor function in the second case. However, retropulsion of bony elements as the cause of cord compression is an indication for surgery given inability of RT to correct bony displacement. Educational efforts should be targeted at reinforcing the importance of initial surgical management by a knowledgeable surgeon to dissipate confusion regarding optimal treatment of such patients. This can be done through various forums such as at national meetings, continuing medical education activities, and by reinforcing the importance of familiarity with consensus guidelines such as those published by the ACR.
Timely management of MSCC leads to improved neurologic outcomes. There are data demonstrating that the most important prognostic factor for ambulatory function after treatment is pretreatment motor function. Therefore, early identification of MSCC and prompt treatment, prior to onset of motor dysfunction, is critical to optimizing post-treatment function and quality of life (2). The majority of surveyed ROs (82.3%) report that less than half of MSCC patients seen in their practices have lost ambulatory function at the time of cord compression diagnosis. Only one respondent (1.6%) reported never seeing loss of ambulation from MSCC. There are literature suggesting that while early diagnosis of MSCC has improved over the years, a significant proportion of patients have already lost ambulation at the time of MSCC treatment (11-13). Spinal cord compression should be suspected in patients with metastatic disease and back pain and further work-up initiated prior to onset of complications such as motor weakness and incontinence. Earlier diagnosis and emergent treatment is needed to minimize the risk of these late complications.
Interestingly, respondents endorse more timely treatment when RT versus neurosurgery is the initial treatment. Since there are randomized data demonstrating the benefit of decompressive surgery prior to RT versus RT alone, efforts should be directed at improving timeliness of neurosurgical intervention in patients recommended for surgery (9,14). While data evaluating impact of time to surgical decompression are sparse, there is evidence that surgery within 48 hours leads to better neurologic outcomes than surgery done greater than 48 hours from onset of neurologic symptoms (14). If decompressive surgery cannot be performed in a timely manner, initial management with RT should be considered as a temporizing measure since delays in treatment can result in irreversible paralysis and/or incontinence (11-13). Further study is required to evaluate how timeliness of treatment is impacted by therapy with upfront surgery versus RT to determine if upfront RT should be considered more strongly in the setting of a potential delay to surgical decompression.
As a cross-sectional survey, results of this study depend on the accuracy and objectivity of respondent recalls. It is possible that responses reflect perceptions or ideal practice rather than actual practice. A chart review of MSCC patients treated within the VHA could help determine how well the survey results reflect actual practice. Similar survey studies and chart reviews in community practice could shed further light on areas for improvement in management of MSCC and how use of the ACR guidelines and other educational activities could address existing gaps in knowledge.
Conclusions
Most VHA ROs are aware of the guideline-recommended management of MSCC, but there exist a few gaps that can be addressed with targeted education and reinforcement. In particular, the role of surgical decompression in the setting of MSCC secondary to bony retropulsion needs reinforcement. Further research is needed to evaluate for treatment delays when the initial treatment is surgery versus RT and to determine the impact of these delays on outcomes. In particular, an increased role for RT should be considered and studied further when neurosurgical services are not readily available. Similar studies are required to determine whether trends noted by VHA ROs are consistent with community practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: Abstract accepted for presentation at 2015 American Society for Radiation Oncology (ASTRO) meeting.
Ethical Statement: Approval for this study of VHA physicians was obtained through the VHA central radiation oncology office in Richmond, VA, USA.
References
- Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br 2010;92:1054-60. [Crossref] [PubMed]
- Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008;7:459-66. [Crossref] [PubMed]
- Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC. Spine (Phila Pa 1976) 2016;41 Suppl 20:S224-30. [Crossref] [PubMed]
- Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, et al. ACR Appropriateness Criteria® Spinal Bone Metastases. J Palliat Med 2013;16:9-19. [PubMed]
- Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Ryu S, et al. ACR Appropriateness Criteria® Metastatic Epidural Spinal Cord Compression and Recurrent Spinal Metastasis. J Palliat Med 2015;18:573-84. [Crossref] [PubMed]
- Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys 1995;32:959-67. [Crossref] [PubMed]
- Bach F, Laresen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107:37-43. [Crossref] [PubMed]
- Loblaw DA, Mitera G, Ford M, et al. A 2011 Updated Systematic Review and Clinical Practice Guideline for the Management of Malignant Extradural Spinal Cord Compression. Int J Radiat Oncol Biol Phys 2012;84:312-7. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Sørensen S, Helweg-Larsen S, Mouridsen H, et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomized trial. Eur J Cancer 1994;30A:22-7. [Crossref] [PubMed]
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ 1998;317:18-21. [Crossref] [PubMed]
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol) 2002;14:472-80. [Crossref] [PubMed]
- Rades D, Huttenlocher S, Dunst J, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol 2010;28:3597-604. [Crossref] [PubMed]
- Quraishi NA, Rajagopal TS, Manoharan SR. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur Spine J 2013;22:1383-8. [Crossref] [PubMed]


