Efficacy of the combination neurokinin-1 receptor antagonist, palonosetron, and dexamethasone compared to others for the prophylaxis of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials
Introduction
Chemotherapy-induced nausea and vomiting (CINV), a common side effect of systemic anticancer therapy, can substantially impair a patient’s quality of life, interfere with a patient’s compliance with anticancer therapy, and result in complications such as electrolyte imbalance, dehydration and malnutrition (1-7). Investigating the safety and efficacy of antiemetics is hence crucial to ameliorating outcomes of cancer patients (8).
Two principal neurotransmitters that are involved in the pathogenesis of CINV are serotonin [5-hydroxytrypatmine 3 (5-HT3)] and substance P (9). Serotonin from enterochromaffin cells in the small intestines can bind to 5-HT3 receptors on vagal afferents and induce CINV (10). Substance P found in vagal afferent neurons can initiate signals to the vomiting centre in the lateral reticular formation of the medulla through bindind to neurokinin-1 (NK1) receptors and induce vomiting (11,12). In an effort to reduce the occurrence of CINV, 5-HT3-serotonin antagonists (5-HT3RA) and neurokinin-1 receptor antagonists (NK1RA) have been developed.
Randomized controlled trials (RCTs) have studied several 5-HT3RAs with respect to efficacy (10,13-15); a systematic review and meta-analysis by Popovic et al suggested that palonosetron (PALO) is superior in terms of efficacy and safety when compared to other 5-HT3RAs (16). The combination of multiple antiemetic medications targeting different molecular pathways associated with CINV, in addition to PALO, has become the standard of care of prophylactic treatment.
The guidelines published by the Multinational Association of Supportive Care in Cancer (MASCC) and European Society of Medical Oncology (ESMO) (17), National Comprehensive Cancer Network (NCCN) (18) and American Society of Clinical Oncology (ASCO) (19) recommend the combination of dexamethasone (DEX), and a 5-HT3RA, for prophylaxis of CINV in patients receiving moderately emetogenic chemotherapy (MEC). For those patients undergoing highly emetogenic chemotherapy (HEC) treatment, the addition of a NK1RA to the regimen of DEX and 5-HT3RA has been recommended for prophylaxis of CINV by MASCC/ESMO; ASCO and NCCN recommends a quadruple-regimen consisting of olanzapine (OLAN), NK1RA, DEX and a 5-HT3RA.
Previous RCTs and a meta-analysis have studied whether a triple regimen (NK1RA, PALO and DEX) is superior in terms of efficacy, compared to the two-medication regimen (PALO and DEX) (8,20). The meta-analysis suggested that the triple regimen is statistically superior to DEX and PALO in 11 of 12 CINV endpoints (8). The review consisted of four studies; several new RCTs have been published since the previous meta-analysis. Additionally, it only compared triple-regimen with double-regimen antiemetic treatments, which limited the statistical power of the review. The purpose of this review was to examine the efficacy of NK1RA, PALO and DEX as reported in RCTs compared to any other prophylactic CINV treatments.
Methods
Search strategy
A literature search was conducted in Ovid MEDLINE(R) (1946 to June Week 4 2017), Embase Classic & Embase (1947 to 2017 Week 27), and the Cochrane Central Register of Controlled Trials (May 2017). MeSH terms, Emtree terms and free text keywords such as “neoplasms” “chemotherapy” “nausea” “vomiting” “palonosetron” and “neurokinin 1 receptor antagonist” were used to prompt relevant literature; the search was also limited to English language clinical trials (Table S1). Reference lists of included RCTs and past meta-analyses were also searched. Titles and abstracts were screened to identify studies relevant for full-text review, and full-text review identified studies eligible for this review based on a pre-specified inclusion criterion.

Full table
Selection criteria
Titles and proceeding were selected for inclusion if they suggested that the study: (I) was a randomized trial; (II) had one intervention arm consisting of at least NK1RA, PALO and DEX for the prophylaxis of CINV; (III) relevant study data was extractable.
Studies were included if at least one endpoint [complete response (CR), complete control (CC), no nausea, no vomiting] in the acute, delayed or overall phases was available. The definition of the endpoints for this review are: (I) CR—no emesis and no use of rescue antiemetics; (II) CC—no emesis, no rescue medication and no more than mild nausea; (III) no nausea—no episodes of nausea; (IV) no vomiting—no episodes of vomiting; (V) acute phase—0 to 24 hours after chemotherapy; (VI) delayed phase—24 to 120 hours after chemotherapy; (VII) overall phase—0 to 120 hours after chemotherapy.
Studies were excluded if they were duplicates of articles found in each database, non-original research reports or small trials (<five patients).
Data extraction and endpoints
The primary endpoint was the proportion of patients achieving CR in the acute, delayed and overall phase. Secondary endpoints included the proportion of patients who achieved CC, no nausea and no vomiting in the acute, delayed and overall phases. Studies must have explicitly reported distinct acute, delayed or overall endpoints. Efficacy data from studies with more than two arms were pooled to compare the combination of NK1RA, PALO and DEX to the numerous other interventions. Endpoints from different cycles were not pooled together. The sample size of each intervention arm was calculated from the randomization ratio when the sample size of the arms was not explicitly reported.
Statistical analyses
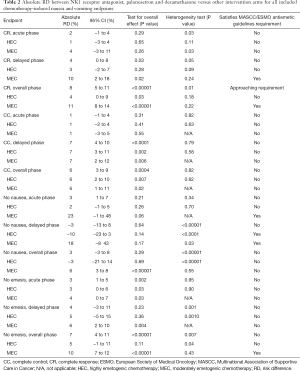
Statistical analyses were performed using Review Manager (RevMan 5.3) by Cochrane IMS. The Mantel-Haenszel method was applied and a random-effects analysis model was used to calculate odds ratio (OR), absolute risk differences (RD) and accompanying 95% confidence intervals (CI). RDs were compared to the 2016 MASCC/ESMO antiemetic guidelines, which noted “as a general rule, the panel considered changes of 10% or greater to be sufficient to warrant the changing of a recommendation” (17).
Trials were stratified based on the authors’ report of chemotherapy emetogenicity – trials comprised of only HEC patients and only MEC patients. Subgroup analyses were conducted within these subgroups to study the efficacy of NK1RA, PALO and DEX; each subgroup had accompanying OR, RD and 95% CI.
Results
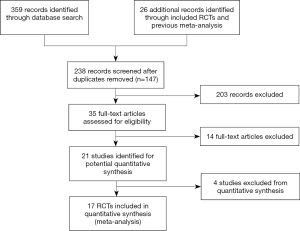
The literature search identified 359 records, and an additional 26 records were identified from the reference lists of included RCTs and previous meta-analyses. After 147 duplicates were removed, a total of 238 title and abstracts were screened for eligibility. Ultimately, 17 RCTs were included in this review (20-36), of which 3,597 patients were randomized to receive NK1RA, PALO and DEX, and 3,438 patients to receive other antiemetic treatments (Figure S1).
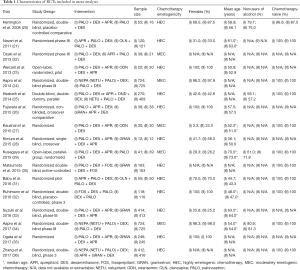
Of the 17 included studies, 12 investigated antiemetic efficacy in patients receiving HEC, while five only studied MEC patients. Four studies recruited exclusively female patients, while four others had the majority of their study population as female patients. Only one study reported the mean age of their population to be less than 50 years old. Ten studies only recruited patients who were chemotherapy-naïve (Table 1).
Full table
CR
The triple antiemetic regimen was not superior to other antiemetic therapies for CINV in the acute phase (OR =1.26, 95% CI: 0.97–1.64). Subgroup analyses of HEC and MEC studies also found a similar conclusion (Figure 1A).
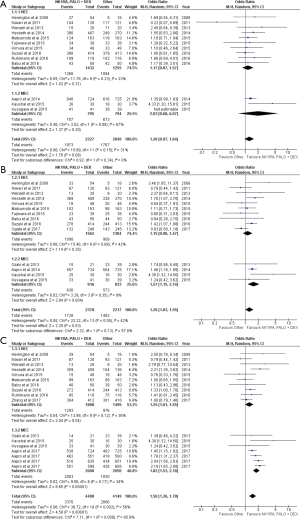
The combination of NK1RA, PALO and DEX was statistically superior to other regimens in the delayed phase (OR =1.26; 95% CI: 1.03–1.55). In HEC studies, the triplet was not statistically superior to other treatments, but the combination was better than other regimens in MEC studies (OR =1.57, 95% CI: 1.15–2.14) (Figure 1B). The RD between the intervention arms in MEC studies satisfied the MASCC/ESMO 10% threshold (Table 2).
Full table
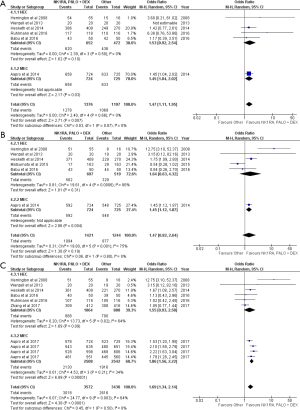
CC
There was no difference in CC between the combination and other intervention arms in the acute phase (OR =1.11, 95% CI: 0.90–1.36) (Figure 2A). The combination was superior to the other antiemetic regimens in the delayed (OR =1.40; 95% CI: 1.20–1.64) (Figure 2B) and the overall (OR =1.32, 95% CI: 1.14–1.54) phases (Figure 2C). These observations were mirrored in the subgroup analyses by emetogenicity. None of the RD values approached the MASCC/ESMO requirement for consideration of revision of guidelines (Table 2).
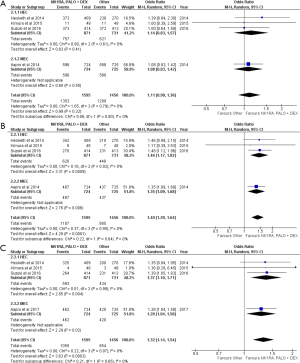
No nausea
NK1RA, PALO and DEX was not superior to other treatments in the acute phase in controlling nausea (OR =1.36, 95% CI: 0.98–1.87). Similarly, there was no difference between the treatment arms in the HEC and MEC settings (Figure 3A). In the delayed phase, there was also no difference in nausea control (OR =0.90; 95% CI: 0.52–1.55). This finding was also similar in the HEC and population (Figure 3B).
The triplet was not statistically similar to other treatments in the overall phase (OR =1.18; 95% CI: 0.90–1.55), and also in the HEC setting (OR =0.88; 95% CI: 0.37–2.11) (Figure 3C). RD analysis indicates that the difference noticed in MEC studies is not considerably large to be studied by MASCC/ESMO anti-emetics panel (Table 2).
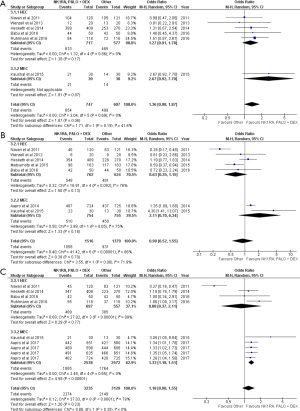
No emesis
NK1RA, PALO and DEX was superior to the other therapies in the acute phase (OR =1.47, 95% CI: 1.11–1.95) (Figure 4A). There was also no difference between the two arms in the delayed phase (OR =1.47, 95% CI: 0.82–2.64) (Figure 4B). The RD for the acute and delayed phases did not pass the 10% MASCC/ESMO requirement (Table 2).
With respect to the overall phase, NK1RA, PALO and DEX was superior (OR =1.69, 95% CI: 1.34–2.14). It was also superior in both the MEC setting (OR =1.86, 95% CI: 1.56–2.22) (Figure 4C). Only RD of MEC studies surpassed the MASCC/ESMO requirement (Table 2).
Discussion
This is the first study to our knowledge that compared the efficacy of the triple-drug antiemetic regimen recommended by MASCC/ESMO (for treatment of CINV in the HEC setting) with any other treatments, as reported in RCTs. Our analyses indicate that the combination is superior to other treatments in all but six endpoints—CC, CR and nausea in the acute phase, nausea and emesis control in the delayed phase, and nausea in the overall phase. However, in almost all of the phase III studies, studies measured nausea as a secondary endpoint and employed different case-definitions.
More importantly, when looking only at HEC and MEC studies, the combination was only superior to others (i.e., 5-HT3RA and DEX; other 5-HT3RA other than PALO with DEX and NK1RA) in three endpoints (overall CR, delayed CC and overall CC) in the HEC setting, which is less than the nine identified endpoints (delayed and overall CR, delayed and overall CC, overall nausea control, and acute, delayed and overall phases for emesis control) in the MEC setting. This observation may be a result of HEC studies simply substituting other 5-HT3RAs in the other treatment arms such as granisetron (GRAN) (23,29,31,34) and ondansetron (24,35), while MEC studies generally completely omitted a class of RAs, such as a NK1RA (20,21,27,28,32), in the comparison arm. The lack of NK1RA, when interpreted in lieu of the fact that the triple-combination arm is more efficacious in the delayed setting for CR, CC, no nausea and no emesis, supports the data that the difference between HEC and MEC studies are a result of the different comparison arms (presence of NK1RA).
The studies of MEC patients that compared a triple antiemetic regimen with a two-antiemetic regimen indicate that the triplet is more efficacious. RD analysis suggests that the combination should be further investigated by the MASCC/ESMO antiemetic guideline panel on the basis that four of the 12 endpoints surpass the 10% threshold. However, not many of our included RCTs documented safety endpoints that could be analyzed using the Mantel-Haenszel method, and more study needs to investigate the safety endpoints for consideration of the panel. Nevertheless, ASCO, NCCN and MASCC/ESMO should investigate into recommending the triple regimen in the MEC setting (adding a NK1RA to the antiemetic regimen), as the results suggest that it is superior to the currently-recommended double-drug regimen.
There are limitations of the present meta-analysis. An inherent limitation of all included RCTs was that differential outcomes between arms for the acute phase may carry-over and affect the results of delayed phase endpoints (37). These endpoints could be explored further in a more controlled setting where antiemetic outcomes on day 1 do not interfere with delayed endpoints. Additionally, some studies were only available for data extraction in abstract form (22,30,35,36). Attempts were made to reach out to authors to see if they could provide more data, not all authors responded.
Conclusions
In conclusion, the combination of NK1RA, PALO and DEX is not inferior to other antiemetic regimens; in fact, the triplet was suggested to be superior in the majority of assessed endpoints. Among MEC patients, in particular, the triple-regimen was suggested to be more efficacious than other regimens that omit NK1RA. Further studies should investigate into the safety of the triple regimen compared to regimens lacking NK1RA, to add to the discussions about whether future CINV prophylaxis guidelines should include NK1RA as a first-line treatment in the MEC setting in addition to the already-suggested HEC setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network, 2015.
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 2011;22:30-8. [Crossref] [PubMed]
- Roscoe JA, Heckler CE, Morrow GR, et al. Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol 2012;30:3389-95. [Crossref] [PubMed]
- Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 2006;24:4472-8. [Crossref] [PubMed]
- Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer 1997;5:307-13. [Crossref] [PubMed]
- Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001-8. [Crossref] [PubMed]
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 2016;24:2381-92. [Crossref] [PubMed]
- Chow R, Popovic M, Chiu L, et al. The combination of NK1 receptor antagonist, palonosetron, and dexamethasone compared to palonosetron and/or dexamethasone for the prophylaxis of chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis of randomized controlled trials. J Pain Manage 2016;9:11-22.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94. [Crossref] [PubMed]
- Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomized, comparative phase III trial. Lancet Oncol 2009;10:115-24. [Crossref] [PubMed]
- Borison HL, McCarthy LE. Neuropharmacology of chemotherapy-induced emesis. Drugs 1983;25:8-17. [Crossref] [PubMed]
- Diemunsch P, Grelot L. Potential of substance P antagonists as anti-emetics. Drugs 2000;60:533-46. [Crossref] [PubMed]
- Gralla R, Lichinister M, Van der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 2003;14:1570-7. [Crossref] [PubMed]
- Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Cancer 2003;98:2473-82. [Crossref] [PubMed]
- Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 2006;17:1441-9. [Crossref] [PubMed]
- Popovic M, Warr DG, DeAngelis C, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 2014;22:1685-97. [Crossref] [PubMed]
- Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119-33. [Crossref] [PubMed]
- Berger MJ, Ettinger DS, Aston J, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw 2017;15:883-93. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Oncol Pract 2017;13:825-30. [Crossref] [PubMed]
- Herrington JD, Jaskiewicz AD, Song J. Randomized, Placebo-controlled, Pilot Study Evaluating Aprepitant Single Dose plus Palonosetron and Dexamethasone for the Prevention of Acute and Delayed Chemotherapy-induced Nausea and Vomiting. Cancer 2008;112:2080-7. [Crossref] [PubMed]
- Navari RM, Gray SE, Kerr AC. Olanzapine versus Aprepitant for the Prevention of Chemotherapy-Induced Nausea and Vomiting: a Randomized Phase III Trial. J Support Oncol 2011;9:188-95. [Crossref] [PubMed]
- Oazaki Y, Horimatsu T, Nozaki A, et al. The efficacy of palonosetron/dexamethasone plus NK1 receptor antagonist (aprepitant) therapy for prevention of chemotherapy induced nausea and vomiting in colorectal cancer patients. Eur J Cancer 2013;49:S524.
- Wenzell CM, Berger MJ, Blazer MA, et al. Pilot study on the efficacy of an ondansetron- versus palonosetron-containing antiemetic regimen prior to highly emetogenic chemotherapy. Support Care Cancer 2013;21:2845-51. [Crossref] [PubMed]
- Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dosed combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328-33. [Crossref] [PubMed]
- Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly-emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 2014;25:1340-6. [Crossref] [PubMed]
- Fujiwara S, Terai Y, Tsunetoh S, et al. Palonosetron versus granisetron in combination with aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer. J Gynecol Oncol 2015;26:311-9. [Crossref] [PubMed]
- Kaushal P, Atri R, Soni A, et al. Comparative evaluation of triplet antiemetic schedule versus doublet antiemetic schedule in chemotherapy-induced emesis in head and neck cancer patients. Ecancermedicalscience 2015;9:567. [Crossref] [PubMed]
- Kimura H, Yamamoto N, Shirai T, et al. Efficacy of triplet regimen antiemetic therapy for chemotherapy-induced nausea and vomiting (CINV) in bone and soft tissue sarcoma patients receiving highly emetogenic chemotherapy, and an efficacy comparison of single-shot granisetron and consecutive-day granisetron for CINV in a randomized, single-blinded crossover study. Cancer Medicine 2015;4:333-41. [Crossref] [PubMed]
- Kusagaya H, Inui N, Karayama M, et al. Evaluation of palonosetron and dexamethasone with or without aprepitant to prevent carboplatin-induced nausea and vomiting in patients with advanced non-small-cell lung cancer. Lung Cancer 2015;90:410-6. [Crossref] [PubMed]
- Matsumoto K, Takahashi M, Sato K, et al. Palonosetron or granisetron for prevention of CINV in patients with breast cancer receiving dexamethasone and fosaprepitant following anthracycline plus cyclophosphamide (AC) regimen. J Clin Oncol 2015;33:9598.
- Babu G, Saldanha SC, Chinnagiriyappa LK, et al. The efficacy, safety, and cost benefit of olanzapine versus aprepitant in highly emetogenic chemotherapy: a pilot study from South India. Chemother Res Pract 2016;2016:3439707. [PubMed]
- Ruhlmann CH, Christensen TB, Dohn LH, et al. Efficacy and safety of fosaprepitant for the prevention of nausea and emesis during 5 weeks of chemoradiotherapy for cervical cancer (the GAND-emesis study): a multinational, randomised, placebo-controlled, double-blind, phase 3 trial. Lancet Oncol 2016;17:509-18. [Crossref] [PubMed]
- Suzuki K, Yamanaka T, Hashimoto H, et al. Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 2016;27:1601-6. [Crossref] [PubMed]
- Aapro M, Karthaus M, Schwartzberg L, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Support Care Cancer 2017;25:1127-35. [Crossref] [PubMed]
- Ogata H, Saito M, Tsuneizumi M, et al. Difference between 1 and 2 generation serotonin receptor antagonists in triplet antiemetic therapy for highly emetogenic chemotherapy in breast cancer patients–according to recent multi-institutional double-blind randomized clinical research on the AC regimen. Cancer Res 2017;77:P5-11-03.
- Zhang L, Lu S, Feng J, et al. Phase 3 study of NEPA versus 3-day oral aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in highly emetogenic chemotherapy (HEC) setting. Support Care Cancer 2017;25:S55-6.
- Roila F, Donati D, Tamberi S, et al. Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer 2002;10:88-95. [Crossref] [PubMed]