Adverse reactions and their management in the treatment of malignant tumors using cytokine-induced killer cells
Introduction
Cytokine-induced killer (CIK) cells are adoptive non-specific immune effector cells, which have a strong tumoricidal activity and high proliferation ability. They can kill multiple tumors at one time (1). Containing multiple cellular components, CIK cells are also referred to as the natural killer (NK)-like T lymphocytes because they express both CD3 and CD56 membrane proteins, and have the strong anti-tumor activities of T lymphocytes and the localized tumoricidal capabilities of non-major histocompatibility complex (MHC) in NK cells, with a killing efficacy of up to 84.7% (2). CIK cell transfusion has become one of the most important adjuvant components in radiotherapy and chemotherapy for cancer patients, which effectively promotes reconstruction of the immune system, elimination of residual disease, and bone marrow purification (3). A total of 1,393 autologous cytokine-induced killer cell transfusion cycles were delivered to 441 patients with malignant tumors in the biological treatment research center of our hospital from January 2012 to December 2012, in conjunction with a range of interventions, such as nursing care and health education. The purpose of this study was to identify the causes and the corresponding management of adverse reactions during the treatment of malignant tumors using cytokine-induced killer cells.
Materials and methods
General information
The present cohort included 441 patients undergoing 1,393 autologous CIK cell transfusion sessions in the biological treatment research center in our hospital from January 2012 to December 2012. One to twelve cycles of the transfusion were delivered for each patient. In total, there were 254 men and 187 women aged 11 to 79 years (with a median age of 56 years), including 75 cases of kidney cancer, 89 cases of liver cancer, 37 cases of prostate cancer, 106 cases of breast cancer, 34 cases of melanoma, 28 cases of lung cancer, and 72 cases other malignancies such as colorectal cancer, bladder cancer, cervical cancer, ovarian cancer, nasopharyngeal carcinoma, and lymphoma.
Preparation and autologous transfusion of CIK
After informed consent was obtained for all patients, 50 mL of anticoagulated peripheral blood was collected to isolate mononuclear cells, which were then incubated with CIK cells containing IFN-γ in 5% CO2 at 37 °C. After 24 h, mouse anti-human CD3 monoclonal antibodies, IL-2, and IL-1α were added in the culture. The cells were passaged approximately every other day, depending on the cell growth, in complete medium with IL-2. After about 14 days of culture, the CIK cells were collected [in a total of approximately (1.0 to 1.3) ×1010], centrifuged, washed with saline, resuspended in 100 mL of normal saline (containing of 5 mL 20% human serum albumin), and sealed (4). The products were then sent to the ward for intravenous transfusion. Each autologous transfusion was considered a cycle of treatment. Blood samples were collected every week for four weeks. Consolidation therapy could be provided every month initially, and later every three, six or twelve months depending on the health condition.
Nursing interventions
Nursing care and health education were provided during the treatment, including care services before, during and after blood collection and transfusion, and those against adverse reactions.
Results
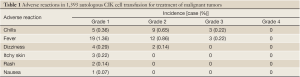
As shown in Table 1, of the 441 patients receiving those 1,393 transfusions, three patients presented chills and a high fever at a temperature over 39.0 °C in 2-4 h after transfusion, which gradually dropped to normal after bed rest, low-flow oxygen, intramuscular injection of phenergan, and oral celecoxib. Twelve patients suffered a moderate fever at a temperature of 38.2-38.9 °C, and nineteen had low fever at a temperature of 37.3-38.0 °C, which was relieved with oral celecoxib or without special treatment. Questionnaires on the life of quality were distributed in 100 randomly selected patients, and 76 responses were received. As shown by the results, 17 patients did not feel significant difference, eight patients experienced disease progression and one had worsened conditions after the transfusion procedure, though the other 50 patients manifested varying degrees of improvement such as increased appetite, better sleep and better mental state.
Discussion
Care before blood collection
Patient assessment and psychological care was provided before blood collection, including the medical condition, state of consciousness, vital signs, treatment, and vascular conditions of each patient. In view that most patients were unfamiliar with CIK cell therapy, and mental disorders could present in such patients, nurses should explain the method and precautions of CIK cell therapy in detail, and demonstrate successful cases to establish the confidence of patients to overcome the disease and eliminate their concerns. Active cooperation with a relaxed and pleasant mood would be particularly critical during the treatment.
Health education was provided before blood collection to inform the patients of the purposes and methods of this procedure so that they would be less nervous and felt relaxed in the supine position. The patients were asked to have a low-fat diet and adequate warm water before the procedure. Any event of dizziness, fatigue, palpitation and other discomfort during the collection should be reported immediately or whenever possible.
Care during blood collection
Each prescription was reviewed by two healthcare providers. A disposable 50 mL syringe was used to inhale 1 mL heparin for anticoagulation, to which a 2.3 cm × 0.8 cm rectangular label was attached along the longitudinal axis indicating the patient name and hospital number. The patient was in a supine or sitting position, with a sterile towel pad beneath the limb to be punctured. The nurse would wear sterile gloves, and perform the procedure following the aseptic techniques strictly. Upon verification of the patient’s identity with the syringe label by two healthcare providers, a 8-gauge scalp needle was used to withdraw 50 mL of venous blood. Upon removal of the syringe, an additional 5 mL air was inhaled into the syringe. The scalp needle was disposed, and a dedicated heparin cap was connected to the tip of the syringe to create a closed environment. The syringe was then gently shaken by rotating 180° to achieve better anticoagulant effects. The collection of anticoagulated peripheral blood for CIK cell therapy did not require storage of the blood samples in a special container. The direct use of a syringe for storage did not only save time and effort of nurses, but also minimized the risk of contamination and hemolysis. No blood contamination or hemolysis occurred in any of the 1,393 procedures.
Health education during blood collection
Withdrawing blood too fast should be avoided. During blood collection, the nurse would talk to the patient in a casual manner to distract his/her attention while observing the condition and facial expression. The patient would be advised against looking at the collection site, and allowed to turn the head away to avoid fainting at the sight of blood and needles due to psychological tensions.
Care after blood collection
After blood collection was completed, the patient’s face color and consciousness were examined, and the patient was inquired of any discomfort such as dizziness and palpitation, in case of transient hypotension due to excessively rapid blood withdrawal. After use, the syringe was directly placed into a sealed bag and sent to the cell preparation room. Aseptic techniques were strictly applied throughout the procedure.
Health education after blood collection
Patients were asked to press their puncture sites for three to five minutes, and were informed that the transfusion would last about 14 d so they would be psychologically prepared. Meanwhile, they were advised to take sufficient rest and drink enough water to replace the blood volume, and to ease psychological tensions as well. Food rich in proteins, vitamins, and calories was provided during the whole period, and due caution was given to prevent colds.
Care during transfusion
When the cells arrived at the ward, the nurse and staff of the cell preparation room would verify the information of those cells, including the name, age, gender, and number of cells, before jointly signing the transfusion registration form. A disposable blood transfusion set was used to filter cell debris (5). Intravenous access was established using 100 mL of normal saline to ensure patency. After verification of the cells and the patient identity by two providers, the cell solution was gently shaken to avoid aggregation, and an additional bottle was used to transfuse the cells. Typically, the transfusion of of 100 mL solution should be completed in 30-50 minutes. A branched tube was used to connect saline and the used cell bottle after the transfusion was completed, so that normal saline traveled into the cell bottle through the tube before being infused to the patient to avoid waste of cells and to ensure more thorough flushing. Aseptic techniques were strictly applied throughout the procedure. Cell transfusion was suspended during radiotherapy and chemotherapy in case the cells were killed and the efficacy compromised during the therapy (6).
Health education during transfusion
The processes, methods and precautions regarding cell transfusion were explained to the patients for maximal cooperation. For those undergoing multiple transfusions, a common participatory nurse-patient relationship could be established taking advantage of their understanding of own physiological functions and state and familiarity with the healthcare staff, to fully mobilize the initiative and enhance the therapeutic effect (7).
Observation of adverse reactions after transfusion
These were the most common adverse reactions, often occurring in 2-6 h after transfusion. Since peripheral blood mononuclear cells were used as autologous CIK cells in the procedure, no graft-versus-host reaction would exist (8). In the present cohort, three patients presented chills and a high fever at a temperature over 39.0 °C in 2-4 h after transfusion, which gradually dropped to normal after bed rest, low-flow oxygen, intramuscular injection of phenergan, and oral celecoxib. Twelve patients suffered a moderate fever at a temperature of 38.2-38.9 °C, and nineteen had low fever at a temperature of 37.3-38.0 °C, which was relieved with oral celecoxib or without special treatment. The causes of fever could be associated with immune response and the use of IL-2, and were mostly self-limiting without other discomfort. Some patients might experience pruritus, rash, difficulty in breathing, palpitation, shortness of breath and other allergic symptoms after transfusion. In such cases, the treatment should be immediately terminated, and the patients could be treated with bed rest, low-flow oxygen, and pacification. Anti-allergy drugs could be used upon prescription. In the present cohort, two patients experienced mild skin itching with rash, which was relieved by oral loratadine. The incidence of such adverse reactions was low, as only 0.29% (4/1,393) of patients had grade 1/3 dizziness, and 0.07% (1/1,393) had grade 1/3 nausea. All of those patients improved after bed rest.
Care and health education after transfusion
After completion of the CIK transfusion, the nurse should connect the cell bottle with a 100 mL saline pack using the branch tube, and flush the residual cells before transfusing them to the patient to avoid wastage. After the end of treatment, patients and their families would be educated in regard to such treatment-related knowledge as the follow-up visit time, appointment for the next treatment, rest and diet.
In conclusion, the best timing of the CIK cell therapy for cancer patients is when the tumor burden, or the number of tumor cells, reaches the minimal level after the end of surgery, chemotherapy and radiation therapy. By eliminating the residual tumor cells, the CIK cell therapy helps consolidate the therapeutic effects, prevent or delay tumor recurrence, and improve the quality of life. The overall disease control of this treatment is 87.8% (9). Of the 1,393 courses of CIK cell treatment, adverse reactions consist mainly of varying degrees of chills and fever, with a low incidence (Table 1). All cases of fever were transient and did not recur after the body temperature was controlled. The use of dexamethasone and other hormones should be avoided so as not to cause immune suppression that may affect the therapeutic effect. RCA analysis of the care quality was conducted for three cases of fever in this study. As shown in the results, our nurses did not violate the standard practice; the saline and disposable blood transfusion tubes used for transfusion were qualified products; and all cell cultures before the transfusion were negative. At last, it was confirmed that the cause of fever was related to the physical conditions of those patients, as well as immune responses. According to the collected questionnaires from the present cohort, 65.8% (50/76) patients reported improved quality of life. Autologous CIK cell transfusion is a relatively safe and effective treatment in clinical settings (10). Successful treatment will rely on sufficient assessment of the conditions, psychological care, and dietary guidance before the procedure; strict implementation of aseptic techniques, and guaranteed cell quality and nursing care during treatment; and close observation and active treatment of adverse reactions (11).
Full Table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mesiano G, Todorovic M, Gammaitoni L, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther 2012;2:673-84. [PubMed]
- Ponte M, Bertone S, Vitale C, et al. Cytokine-induced expression of killer inhibitory receptors in human T lymphocytes. Eur Cytokine Netw 1998;1:69-72. [PubMed]
- Liu QX, Shao XZ, Sun LX. Research development of Cytokine-induced killer cells in the treatment of tumor. Cheng De Yi Xue Yuan Xue Bao 2007;2:187-9.
- Huang LX, Zhou QM, Xia JC. Evaluating the safety and efficacy of minimally invasive surgery combined with autologous CIK cells in the treatment of primary hepatic carcinoma. Guang Dong Yi Xue 2007;2:1466-8.
- Lin HF, Lin HY, Lin YK. Nursing care of patients with malignant tumors treated with adoptive immunotherapy with CIK. Hu Li Yan Jiu 2005;2:1006-7.
- Zhong LL, Wu YD. Nursing of malignant tumor patients treated with immunotherapy with Cytokine-induced killer cells. Xi Nan Jun Yi 2010;2:1030.
- Quan HQ. Nursing care and health education of cancer patients treated with autologous CIK cell transfusion. Jia Ting Hu Li 2008;1:1046-7.
- Tong FJ. Nursing care of 43 cases of malignant tumor receiving cellular immunotherapy. Dang Dai Hu Shi 2011;1:64-5.
- Guan HM. Clinical observation of adoptive immunotherapy with autologous Cytokine-induced killer cells (CIK) for malignant tumor. Zhong Guo Xian Dai Yao Wu Ying Yong 2011;1:68.
- Ma Y, Zhang Z, Tang L, et al. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy 2012;2:483-93. [PubMed]
- Xiao SJ. Chinese and western medicine nursing interventions on CIK cell treatment of malignant tumors. Lin Chuang Yi Xue Gong Cheng 2012;2:2014-5.


