Palliative care for Parkinson’s disease
Background
Palliative care is an approach that improves the quality of life of patients and their families facing the problem associated with all life-threatening illnesses. Palliative care is important in neurology as the trajectory of many neurological conditions is progressive and incurable (1). Patients with Parkinson’s disease (PD) are known to have motor and non-motor symptoms which become refractory to treatment with time, associated caregiver distress and increased mortality. Palliative care seeks to reduce suffering in PD patients and their families through physical, psychosocial and spiritual support. Applying palliative care in PD has been specifically addressed in international symposium (2), by professional society (3) and in national guidelines (4,5).
Disease burden and mortality in PD
PD is a slowly progressive multi-system neurodegenerative disorder, mainly affecting patients in later years of life (6). It is the second most common neurodegenerative disease worldwide, with its incidence and prevalence on the rise along with aging of the population (7).
The characteristic classical motor features (the parkinsonian symptoms) of PD include bradykinesia, rigidity, resting tremor and postural instability. Patients with PD also suffered from multiple non-motor features including olfactory dysfunction, cognitive impairment, psychiatric symptoms, sleep disorders, autonomic dysfunction, pain and fatigue (6). As the disease progresses, both motor and non-motor symptoms become prominent and treatment-resistant. Advanced PD is identified by disability requiring help for the activities of daily living, presence of motor fluctuations with limitations to perform basic activities of daily living without help, severe dysphagia, recurrent falls, and dementia (8). Up to now there are no disease-modifying treatments that can stop or delay the disease process or mortality.
Patients with PD were found to have more physician consultations and more emergency department visits per year than did reference subjects of similar age and sex in a population-based study (9). They also have greater and earlier need for institutional care (9). A cross-sectional analysis of a hospital admission data involving more than 180,000 patients with PD found that they had more hospital admissions compared with patients without PD (10). The main reasons for admission were pneumonia (13.5%), motor decline (9.4%), urinary tract infection (9.2%) and hip fractures (4.3%), and they occurred 1.5 to 2.6 times more frequently in patients than controls. They were almost twice as likely to be hospitalized for more than 3 months (ratio 1.90, 95% CI: 1.83–1.97) and more likely have in-hospital death (ratio 2.46, 95% CI: 2.42–2.49).
The mortality risk among PD patients is shown to by increased by 1.5–2.2-fold in two meta-analyses (11,12). Increasing age and presence of dementia were most commonly associated with increased mortality (11,12). In post-mortem studies, mean duration of disease until death ranged from 6.9 to 14.3 years (11). Data on cause of death from retrospective review on death certification suggested that patients with PD are more commonly died from dementia, pneumonia and other infections, as compared with general population in which death from cancer and ischaemic heart disease is more prevalent (13,14).
Starting palliative care in patients with PD
Patients with PD benefit early from palliative care in view of the impact of the disease impairing autonomy and quality of life. The provision of palliative care in patients with PD focuses on unmet needs and should be aligned with patient priorities. It is recommended that a palliative care approach should be applied from the early phase, throughout the course of the disease, complementing but not replacing other treatments (5). However, like other patients with chronic neurological condition, the individual needs may vary over time, therefore it is suggested that a model of dynamic involvement of palliative care services should be adopted (15). The services can be triggered at times of particular symptoms or psychosocial issues—such as the start of new interventions (e.g., artificial nutrition) or at the very end of life.
For patients with complex physical, social, psychological and/or spiritual needs that do not respond to simple or established protocols of palliative care, there should be access to the support from specialist palliative care service (16,17).
However, rate of use of hospice in PD patients has been low (18,19). Caregivers often considered palliative care services to be synonymous with hospice care, and hence they did not consider this service option (20). Health care workers also have uncertainty about timing of palliative care, such that it was often not introduced until a crisis point (21).
Prognostication in PD
A way of identifying patients with palliative care needs is by prognostication. Pulling together a range of clinical, social and other factors that give a whole picture of deterioration, prognostication by a health care profession can be done intuitively by a surprise question “would you be surprised if the patient were to die in the next year, months, weeks, days?” (22) A negative answer should prompt palliative measures that might be taken to improve the patient’s quality of life and in preparation for possible further decline (22). On the other hand, some general markers of advanced disease may also prompt clinician the need of palliative care for a PD patient, including weight loss, declining functional status, frequent infections and hospitalizations, skin breakdown and evidence of malnutrition (23). Another simple estimation is by using the palliative performance scale (PPS) which measures the functional status of a patient. PPS has been shown to guide prognostication (24) and it is used to determine the eligibility for enrolment in palliative care benefits program (25).
Currently there are no specific tools to predict prognosis in PD. A study aiming at recognition of hospice eligibility for PD tried to identify variables which have a higher probability of occurring uniquely in 6 to 12 months before death when compared to 18 to 24 months before death (26). The results suggested that body mass index less than 18 kg/m2, accelerated weight loss and a reduction in prescribing of dopaminergic medications as side effects outweigh benefit are the specific predictors.
On the other hand, there are specific guidelines to prompt earlier identification of patients with advanced PD and likely limited survival (Table 1).

Full table
Symptom burden in advanced PD
There are established guidelines in management of motor (4,28-31) and non-motor (4,28,31-34) symptoms in PD. However, symptom burden in advanced PD is still high, and it has been reported to be of similar degree as in metastatic cancer (35).
The motor symptoms of PD progress over years and the Hoehn and Yahr scale is a commonly used system for describing this (Table 2) (36). After the honeymoon period in early years of PD when antiparkinsonian drugs usually provide excellent control over the motor symptoms of bradykinesia and rigidity, majority of patients begin to experience less reliable drug response upon disease progression. Patient would experience complications from the long-term drug treatment, including motor fluctuations (wearing-off and on-off symptoms) and dyskinesia. In advanced PD, prominent motor symptoms, as reported in a cohort of 50 patients with stage 4 or 5 of Hoehn and Yahr, include severe akinesia, postural instability, freezing of gait, dysarthria and dysphagia (37).
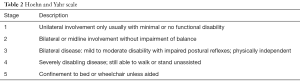
Full table
Both motor and non-motor symptoms are prevalent in advanced PD. Using a checklist with 20 symptoms relevant in palliative care, a cross-sectional community study on 85 patients with PD stages 3–5 Hoehn and Yahr reported a mean of 10.7 physical symptoms (38,39). Pain, fatigue, daytime somnolence and problems with mobility were found in more than 80% of PD patients. More than half of patients also had constipation, loss of bladder control, swallowing difficulties, drooling, breathlessness and sleep problems. Among these symptoms, pain, fatigue, constipation and drooling were rated as causing severe problems. Anxiety and depression were also reported in 70% and 60% of patients (39).
From the patient’s perspective, there is a recent questionnaire survey (40) which included 814 PD patients (70% were of stages 3–5 Hoehn and Yahr) who felt subjectively severely affected by their illness. The commonest reasons for feeling severely affected were mobility impairment (34.9%), coordination problems (17.0%), speech problems (12.2%), and limited day-to-day activities (7.8%), e.g., getting dressed and personal hygiene. Significant associations were observed between subjectively felt severe affectedness and Hoehn and Yahr, poorer health, higher nursing care level, and having no children (40).
There are symptom assessment tools that could help better delineation of the palliative care needs in PD. Palliative care outcome scale (POS) (41) is a 10-item reliable and validated core outcome measure that was designed to cover those domains considered important for palliative care, including pain control, symptom control, patient anxiety, family anxiety, information, sharing feelings, depression, self-worth, practical needs and time wasted (41). POS with additional Parkinsonism Plus symptoms (POS-PP) is a 20-item validated extension of the core POS assessing symptoms (POS-S), with additional Parkinsonism Plus symptoms added (38). Edmonton Symptom Assessment System (ESAS) (42) is commonly used for symptom screening and longitudinal monitoring in patients seen by palliative care in both inpatient and outpatient settings. It has been psychometrically validated and translated into over 20 languages (43). It assesses nine common symptoms including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, wellbeing and shortness of breath. To address PD specific symptoms, there is a modified version of ESAS (ESAS-PD) with coverage of clinically relevant symptoms, including constipation, difficulty swallowing, stiffness and confusion (35). This tool was found to be responsive to treatment, and patients with advanced PD were able to complete ESAS-PD independently or with caregiver assistance (35).
Advance care planning (ACP)
In the definition recently endorsed by the European Association for Palliative Care, ACP is a process enables individuals to define goals and preferences for future medical treatment and care, to discuss these goals and preferences with family and healthcare providers, and to record and review these preferences if appropriate (44). ACP is known to be associated with the end of life wishes more likely to be known and respected, the bereaved family members with less stress, anxiety and depression, and a higher satisfaction in patient and family (45). In view of the impact of PD on cognitive function and communication in its progression, patients should have an opportunity to address on ACP early in the disease. The content of ACP can be more targeted later upon progression of disease.
However, it is recommended that ACP should be adapted to the individual’s readiness to engage in the ACP process (44), as some patients with early stage PD felt that ACP may be too depressing, hoping that a cure would come in time for them (46). In a survey of 267 patients with PD on preferences about prognostic and end of life discussions (47), although 94% of patients preferred early information on prognosis and treatment and 68.5% actually reported having some kind of ACP document, only about half of the patients wanted to discuss advance care document early. A smaller proportion wanted early discussions about end-of-life care planning (27%) or end-of-life care options such as hospice (21%), and a very small number felt end-of-life issues should never be discussed (47). More commonly patients would like to discuss these issues when their disease worsened, therefore it is recommended that potential triggers for initiation of ACP should be identified and developed (44). In the case of PD, these could be progression of disease in terms of their motor and non-motor features (4), deterioration in functioning and transitions in care.
ACP usually involves patient and their important ones, usually their family/caregivers, and the health care team. Though patients would agree on their own responsibility to bring up discussions on issues of life expectancy, end-of-life care planning, and end-of-life care options such as hospice (47), they might not know to whom they should turn for ACP (48). Some thought that their neurologist should raise these topics (47), while some with advance directives did not include their physician in the process and believed it should be done by the family or a lawyer (48). Caregivers of PD patients may wish the health care professional to have greater input to inform the ACP. In a study of 64 spouses of patients with PD (49), while most (92%) believed that they would be involved in the decision making, 70% also thought that physicians should be involved.
Caregiver needs and support
A caregiver refers an individual who provide ongoing care and assistance, without pay, for family members and friends in need of support due to physical, cognitive, or mental health conditions (50). Family caregivers are the greatest support of patients in advanced diseases especially when they wish to be cared for at home. On top of the caregiving role, the multiple roles played by the caregiver of palliative care patients include well-being enhancer, “handyman” in daily tasks, minimizer/manager of suffering, palliative care facilitator and responsible for the continuity of care, learner in care provision and decision-maker at the end-of-life (51).
While many PD patients felt their families were anxious or worried about them (39), most PD caregivers themselves indeed felt unprepared for their role (52). Particular challenges to PD caregiver are the cognitive, personality and behavioral changes that may occur, especially in advanced stages of disease (5).
A meta-analysis on factors associated with PD caregiver distress integrated findings from ten studies on the correlates of caregiver distress in terms of depressive symptoms, burden, as well as stress induced by the caregiving role (53). Motor symptoms of patients with PD were found to have the strongest relationship with caregiver distress. Increased motor symptoms and higher dependency in activities of daily living showed the highest effect sizes on caregiver distress (|r| = 0.42–0.43) than did patients’ higher level of depression (r= 0.37), more advanced disease stage (r = 0.33), longer duration of disease (r= 0.31), as well as poorer cognitive functioning level (|r|=0.28). On the contrary, a systematic review on predictive factors of psychosocial outcomes in caregivers of PD patients found that psychological and non-motor symptoms appear to be more important than physical symptoms and levels of disability (54).
So far research on effective interventions for caregiver of patients with PD is limited and inconclusive (2). A systematic review by Hempel et al. (55) evaluated 30 studies (24 full studies and 6 studies published as abstracts) to identify and examine the evidence on psychosocial interventions for caregivers. Day care, night-sitting services, community care assessment, web-based instructional videos on caregiving tips/strategies, formal education classes, and support groups are identified intervention. However, the clinical or cost effectiveness could not be assessed in most of the intervention because of weak research designs, small numbers of participants and inconsistent results. On the other hand, a psychosocial intervention of eight weekly sessions of 90-minute duration, the Patient Education Program Parkinson (PEPP) (56), was shown to benefit PD caregivers in a randomized controlled trial. A significant effect for the caregivers on psychosocial problems and need for help was found. Patients’ and caregivers’ mood also improved significantly after each session.
Though at the mean time research on effective caregiver interventions in PD is pending, caregivers can benefit from information in internet resource (e.g., Parkinson’s UK https://www.parkinsons.org.uk/, National Parkinson Foundation http://www.parkinson.org/) and local social agents which provide generic support to caregiver. Referral to a clinical psychology may help caregiver in emotional distress. Bereavement care is also an integral part of the support to caregiver in end-of-life care.
Multidisciplinary approach
Teamwork is the most effective way to accomplish complex tasks. Because of the multifaceted needs of patients with PD, a multidisciplinary team (MDT) approach should be adopted and delivered in a coordinated manner.
An MDT in PD has combination of different members which might include neurologist, general practitioner, palliative care specialist, gerontologist, rehabilitative specialist, PD nursing specialist, physiotherapist, occupational therapist, speech therapist, dietitian, psychologist, pharmacist, social workers, spiritual care workers and voluntary organizations, as well as support from various specialists including gastroenterologist, neurosurgeon, sleep specialist and urologist (5,57,58). Despite the diverse disciplines, an ideal model would be an “interdisciplinary” approach which is based on synergistic and interdependent interaction of team members, like a hand able to achieve more than total of each individual finger can alone (59). Also it is important to have a clear, defined pathway to follow when an individual needs help or advice and to have a single point of contact, i.e., a “key worker(s)” (5). This key worker can be a PD nursing specialist in all stages of PD (58). For PD patients in end-of-life phase who preferred to die at home, the key worker may be a community hospice nurse (60). Caregivers should also be included in the teamwork. Engaging caregivers in understanding of disease and deciding of treatment could contribute to greater satisfaction of care and improve adherence to therapy (57).
A recent review of evidence of MDT interventions in people with PD (57) has covered 13 studies including 6 RCTs. The studies involved patients with both early and late stage by Hoehn and Yahr staging of disability in PD. In general, MDT approach was shown to improve the health-related quality of life and motor function for patients with PD and quality of life of their caregivers. The limitations of the review include the lack of a systematic literature search and the limited number of controlled studies.
Conclusions
A palliative care approach should be introduced early in the course of PD. Palliative care for PD should be need-based, focusing on improving of QOL and autonomy, ACP and support to caregiver. An ideal model of care would be interdisciplinary team providing clear care pathway and a single point of contact. Future directions should include development of tools to guide prognostication and referral to specialist palliative care, assessment of specific palliative care intervention for patients with PD and model to guide caregiver assessment and support.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Palliative care in neurology. The American Academy of Neurology Ethics and Humanities Subcommittee. Neurology 1996;46:870-2. [PubMed]
- Kluger BM, Fox S, Timmons S, et al. Palliative care and Parkinson's disease: Meeting summary and recommendations for clinical research. Parkinsonism Relat Disord 2017;37:19-26. [Crossref] [PubMed]
- International Parkinson and Movement Disorder Society. Task Force on Palliative Care. Accessed 1 December 2017. Available online: https://www.movementdisorders.org/MDS/About/Committees--Other-Groups/MDS-Task-Forces/Task-Force-on-Palliative-Care.htm
- NICE guideline (2017). Parkinson’s disease in adults. Accessed 3 December 2017. Available online: https://www.nice.org.uk/guidance/ng71
- The Irish Palliative Care in Parkinson’s Disease Group (2016). Palliative care in People with Parkinson’s disease: Guidelines for professional healthcare workers on the assessment and management of palliative care needs in Parkinson’s disease and related Parkinsonian syndromes. Cork: University College Cork. Accessed 1 December 2017. Available online: https://www.ucc.ie/en/media/research/parkinsonscare/PalliativecareinPeoplewithParkinsonsdisease.pdf
- Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896-912. [Crossref] [PubMed]
- Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014;29:1583-90. [Crossref] [PubMed]
- Luquin MR, Kulisevsky J, Martinez-Martin P, et al. Consensus on the Definition of Advanced Parkinson's Disease: A Neurologists-Based Delphi Study (CEPA Study). Parkinsons Dis 2017;2017. [Crossref] [PubMed]
- Parashos SA, Maraganore DM, O'Brien PC, et al. Medical services utilization and prognosis in Parkinson disease: a population-based study. Mayo Clin Proc 2002;77:918-25. [Crossref] [PubMed]
- Low V, Ben-Shlomo Y, Coward E, et al. Measuring the burden and mortality of hospitalisation in Parkinson's disease: A cross-sectional analysis of the English Hospital Episodes Statistics database 2009-2013. Parkinsonism Relat Disord 2015;21:449-54. [Crossref] [PubMed]
- Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014;29:1615-22. [Crossref] [PubMed]
- Xu J, Gong DD, Man CF, et al. Parkinson's disease and risk of mortality: meta-analysis and systematic review. Acta Neurol Scand 2014;129:71-9. [Crossref] [PubMed]
- Fall PA, Saleh A, Fredrickson M, et al. Survival time, mortality, and cause of death in elderly patients with Parkinson's disease: a 9-year follow-up. Mov Disord 2003;18:1312-6. [Crossref] [PubMed]
- Beyer MK, Herlofson K, Arsland D, et al. Causes of death in a community-based study of Parkinson's disease. Acta Neurol Scand 2001;103:7-11. [Crossref] [PubMed]
- The National End of Life Care Programme (NEoLCP) and the Neurological Alliance (2010). End of life care in long term neurological conditions: a framework for implementation. Accessed 1 December 2017. Available online: https://www.mssociety.org.uk/sites/default/files/Documents/Professionals/End%20life%20care%20long%20term%20neuro%20conditions.pdf
- Palliative Care Australia (2005). A Guide to Palliative Care Service Development: A population based approach, PCA, Canberra. Accessed 1 December 2017. Available online: https://www.caresearch.com.au/caresearch/Portals/0/Documents/NSAP/Defining%20your%20SPCS.pdf?ver=2012-12-21-140658-850
- Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med 2013;368:1173-5. [Crossref] [PubMed]
- Moens K, Houttekier D, Van den Block L, et al. Place of death of people living with Parkinson's disease: a population-level study in 11 countries. BMC Palliat Care 2015;14:28. [Crossref] [PubMed]
- Snell K, Pennington S, Lee M, et al. The place of death in Parkinson's disease. Age Ageing 2009;38:617-9. [Crossref] [PubMed]
- McLaughlin D, Hasson F, Kernohan WG, et al. Living and coping with Parkinson's disease: perceptions of informal carers. Palliat Med 2011;25:177-82. [Crossref] [PubMed]
- Fox S, Cashell A, Kernohan WG, et al. Interviews with Irish healthcare workers from different disciplines about palliative care for people with Parkinson's disease: a definite role but uncertainty around terminology and timing. BMC Palliat Care 2016;15:15. [Crossref] [PubMed]
- The GSF Proactive Identification Guidance (PIG) 2016 vs6 © The Gold Standards Framework Centre in End of Life Care. Accessed 1 December 2017. Available online: http://www.goldstandardsframework.org.uk/cd-content/uploads/files/PIG/NEW%20PIG%20-%20%20%2020.1.17%20KT%20vs17.pdf
- Lugassy M. A palliative care approach to Parkinson’s and other neurodegenerative diseases. National Hospice and Palliative Care Organization Palliative Care Resource Series. Copyright © 2016 National Hospice and Palliative Care Organization.
- Lau F, Downing M, Lesperance M, et al. Using the Palliative Performance Scale to provide meaningful survival estimates. J Pain Symptom Manage 2009;38:134-44. [Crossref] [PubMed]
- Palliative care benefits program. BCMJ, Vol. 53, No. 8, October, 2011, page(s) 431 — Pulsimeter. Accessed 1 December 2017. Available online: http://www.bcmj.org/pulsimeter/palliative-care-benefits-program
- Goy ER, Bohlig A, Carter J, et al. Identifying predictors of hospice eligibility in patients with Parkinson disease. Am J Hosp Palliat Care 2015;32:29-33. [Crossref] [PubMed]
- Boersma I, Miyasaki J, Kutner J, et al. Palliative care and neurology: time for a paradigm shift. Neurology 2014;83:561-7. [Crossref] [PubMed]
- Scottish Intercollegiate Guidelines Network (2010). SIGN guideline 113: Diagnosis and pharmacological management of Parkinson’s disease. A national clinincal guideline. NHS Quality Improvement Scotland. Accessed 1 December 2017. Available online: http://www.sign.ac.uk/assets/sign113.pdf
- Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S2-41. [Crossref] [PubMed]
- Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983-95. [Crossref] [PubMed]
- Oertel WH, Berardelli A, Bloem BR, et al. Late (complicated) Parkinson’s disease. In: Gilhus NE, Barnes MP, Brainin M. editors. European Handbook of Neurological Management 2nd ed. Oxford, UK: Wiley-Blackwell Publishing Ltd., 2011:237-67.
- Miyasaki JM, Shannon K, Voon V, et al. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996-1002. [Crossref] [PubMed]
- Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:924-31. [Crossref] [PubMed]
- Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S42-80. [Crossref] [PubMed]
- Miyasaki JM, Long J, Mancini D, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinsonism Relat Disord 2012;18 Suppl 3:S6-9. [Crossref] [PubMed]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42. [Crossref] [PubMed]
- Coelho M, Marti MJ, Tolosa E, et al. Late-stage Parkinson's disease: the Barcelona and Lisbon cohort. J Neurol 2010;257:1524-32. [Crossref] [PubMed]
- Higginson IJ, Gao W, Saleem TZ, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLoS One 2012;7. [Crossref] [PubMed]
- Saleem TZ, Higginson IJ, Chaudhuri KR, et al. Symptom prevalence, severity and palliative care needs assessment using the Palliative Outcome Scale: a cross-sectional study of patients with Parkinson's disease and related neurological conditions. Palliat Med 2013;27:722-31. [Crossref] [PubMed]
- Strupp J, Kunde A, Galushko M, et al. Severely Affected by Parkinson Disease: The Patient's View and Implications for Palliative Care. Am J Hosp Palliat Care 2018;35:579-85. [PubMed]
- Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 1999;8:219-27. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J Pain Symptom Manage 2017;53:630-43. [Crossref] [PubMed]
- Rietjens JA, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol 2017;18:e543-51. [Crossref] [PubMed]
- Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. [Crossref] [PubMed]
- Fox S, Cashell A, Kernohan WG, et al. Palliative care for Parkinson's disease: Patient and carer's perspectives explored through qualitative interview. Palliat Med 2017;31:634-41. [Crossref] [PubMed]
- Tuck KK, Brod L, Nutt J, et al. Preferences of patients with Parkinson's disease for communication about advanced care planning. Am J Hosp Palliat Care 2015;32:68-77. [Crossref] [PubMed]
- Boersma I, Jones J, Carter J, et al. Parkinson disease patients' perspectives on palliative care needs: What are they telling us? Neurol Clin Pract 2016;6:209-19. [Crossref] [PubMed]
- Kwak J, Wallendal MS, Fritsch T, et al. Advance care planning and proxy decision making for patients with advanced Parkinson disease. South Med J 2014;107:178-85. [Crossref] [PubMed]
- Canadian Hospice Palliative Care Association. Fact Sheet: The Role of Family and Informal Caregivers. Accessed 1 December 2017. Available online: http://www.chpca.net/media/153773/caregiver_day_-_fact_sheet.pdf
- Reigada C, Pais-Ribeiro JL, Novellas A, et al. The Caregiver Role in Palliative Care: A Systematic Review of the Literature. Health Care Curr Rev 2015;3:143.
- Goy ER, Carter JH, Ganzini L. Needs and experiences of caregivers for family members dying with Parkinson disease. J Palliat Care 2008;24:69-75. [PubMed]
- Lau KM, Au A. Correlates of Informal Caregiver Distress in Parkinson's Disease: A Meta-Analysis. Clinical Gerontologist 2011;34:117-31. [Crossref]
- Greenwell K, Gray WK, van Wersch A, et al. Predictors of the psychosocial impact of being a carer of people living with Parkinson's disease: a systematic review. Parkinsonism Relat Disord 2015;21:1-11. [Crossref] [PubMed]
- Hempel S, Norman G, Golder S, et al. Psychosocial interventions for non-professional carers of people with Parkinson's disease: a systematic scoping review. J Adv Nurs 2008;64:214-28. [Crossref] [PubMed]
- A'Campo LE, Wekking EM, Spliethoff-Kamminga NG, et al. The benefits of a standardized patient education program for patients with Parkinson's disease and their caregivers. Parkinsonism Relat Disord 2010;16:89-95. [Crossref] [PubMed]
- Pedersen SW, Suedmeyer M, Liu LW, et al. The role and structure of the multidisciplinary team in the management of advanced Parkinson's disease with a focus on the use of levodopa-carbidopa intestinal gel. J Multidiscip Healthc 2017;10:13-27. [Crossref] [PubMed]
- Qamar MA, Harington G, Trump S, et al. Multidisciplinary Care in Parkinson's Disease. Int Rev Neurobiol 2017;132:511-23. [Crossref] [PubMed]
- Crawford GB, Price SD. Team working: palliative care as a model of interdisciplinary practice. Med J Aust 2003;179:S32-4. [PubMed]
- Shepperd S, Goncalves-Bradley DC, Straus SE, et al. Hospital at home: home-based end-of-life care. Cochrane Database Syst Rev 2016;2. [PubMed]


