Changes in laser-evoked potentials during hypnotic analgesia for chronic pain: a pilot study
Introduction
In 2015, the Society of Psychological Hypnosis, Division 30 of the American Psychological Association, formulated a new definition of hypnosis: hypnosis is “A state of consciousness involving focused attention and reduced peripheral awareness characterized by an enhanced capacity for response to suggestion” (1). Hypnotic processes can modify both intrinsic (self-awareness) and extrinsic (environmental awareness) brain networks, particularly the perception of sensory events (2-4). With advances in functional neuroimaging, the near future is expected to bring an increase in the number of studies on altered (modified) states of consciousness and on the use of hypnosis and suggestion to modulate subjective experience and gain insights into normal and abnormal cognitive functioning.
A key feature of hypnosis is its ability to induce altered states of consciousness. Findings from cognition and neuroimaging studies have documented the power that suggestion wields over attention functions and associated brain networks (5-8). Furthermore, experiments have shown that attention, expectation, and hypnosis may affect information processing in the human brain (6,9-11). Both endogenous top-down control and exogenous bottom-up capture of attention enhance performance by increasing the neural activity in a given sensory system (11). Focusing on the role of top-down control processes, Landry and coworkers studied the involvement of intrinsic brain networks in cognitive control and self-referential cognition, including the executive, salience, and default networks. They described how these neural dynamics may relate to contemporary theories of hypnosis and showed that hypnosis correlates with activation of the lingual gyrus, a brain region involved in higher-order visual processing and mental imagery (9). In their critical review and quantitative meta-analysis of neuroimaging assessment of the reliability of brain activity patterns associated with hypnosis, they found that, despite the abundance of neuroimaging studies, there is no solid consensus on the neural mechanisms subtending hypnosis. Its multifaceted nature, combined with lack of methodological standards in the field, likely account for this heterogeneity of results.
Moreover, the neural mechanism underlying the antinociceptive effects of hypnosis also remain unclear. Recent neurophysiological studies on pain have shown that there is no single “pain center” in the brain, but rather that areas of the peripheral and central nervous systems interact with one another, each of which contributing to the overall experience of pain. The cortical areas most often activated during pain are the thalamus, anterior cingulate cortex (ACC), insular cortex, sensory cortices (primary and secondary), and prefrontal cortex. The relative contribution of each area to the pain experience variably depends on the nature of the pain stimuli. Each of the brain areas involved in pain processing has been shown to respond to hypnosis in more than one study, suggesting that hypnotic analgesia may influence different areas of the nervous system involved in pain processing rather than via a single, unique mechanism (12).
The experience of pain comprises affective/emotional and sensory-discriminatory components, each associated with particular neural systems. Some studies indicate that analgesic suggestions can selectively alter any one of these components of the pain experience (13,14). Also, connectivity between different brain areas may result increased or decreased depending on the type of suggestions (15,16). The effect of hypnotic analgesia may depend on such various factors as wording of hypnotic suggestions, suggestible (also genetic) trait (high or low hypnotizability) (7,17), and hypnotic induction (18), although the question remains open about the relative importance of induction (9).
Clinical hypnosis is considered a psychological intervention and a complementary, alternative and integrative medicine in pain therapy and palliative care (19-22). It is given as adjuvant therapy to reduce acute and chronic pain in patients with cancer or severe chronic disease and it has been demonstrated as being equal to or more effective than no treatment or standard care or other active treatments (19-25). When combined with local anesthesia and conscious sedation, hypnosis was associated with improved peri-operative comfort of surgical patients and surgeons and faster recovery of patients (23). A recent meta-analysis involving more than 2,000 patients undergoing surgical or medical procedures examined the effects of hypnosis on various pre- and post-operative factors such as emotional distress, pain, medication consumption, physiological parameters, recovery, and surgical procedure time as compared to standard care alone (24). The purpose of the present study was to determine the effects of clinical hypnosis as compared to distraction of attention (DA) on laser-evoked signals and pain intensity and unpleasantness.
Methods
Patients
Ten outpatients with chronic pain (mean duration >3 months) were recruited for the study. Their therapy remained unchanged during the 30-day period before assessment. Demographic and clinical data are shown in Table 1.
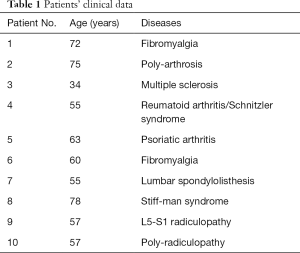
Full table
Exclusion criteria were ongoing depression [as assessed according to “Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition” (DSM-5) criteria], severe cognitive impairment or dementia (Mini Mental State Examination score ≤24), history of psychosis (including hallucinations and delusions), drug or alcohol misuse, opioids use, and previous diagnosis of polyneuropathy or diabetes.
Clinical assessment
Patients were asked to describe the body distribution and qualitative characteristics of their pain symptoms. Pain intensity and unpleasantness (referred to the last 4 weeks) were graded on an 11-point numerical rating scale (NRS) from 0 (no pain) to 10 (worst pain imaginable).
Hypnosis induction
All patients were familiar with the hypnotic procedure. Hypnosis was induced during stimulation with laser-evoked potentials (LEPs). Hypnosis comprised an introduction to the procedure during which the patients were told that suggestions for imaginative experiences would be presented. Hypnotic induction was an extended initial suggestion for using one’s imagination and contained further elaborations of the introduction. During hypnosis, the patients were guided by the hypnotist to respond to suggestions for changes in their subjective experience and perception of pain (26). When the patients were in the hypnotic state, the hypnotist suggested particular visualizations that evoked feelings that modified painful sensation, and thus reduced the perception of pain. The patients were trained under hypnosis how to interpret the pain sensation and to transform it into a feeling of a different nature, for example, light or medium tension, moderate pressure, beneficial warmth or cold. The hypnotist helped the patients visualize themselves in a beautiful place and experience these new sensations. We applied transient anesthesia of one hand, as explained below:
“While in a state of relaxation imagine immersing your hand in a container of melting ice cubes. From the wrist to the tip of your fingers, the ice acts on your hand like a powerful anesthesia…making it less and less sensitive. You will feel your hand becoming increasingly insensitive…and the anesthesia will increase. You will also feel the anesthesia will last until you repeat to yourself three times ‘Everything is normal’”.
DA technique
The therapist read an article from an Italian magazine during LEP stimulation to distract the patients. This distraction technique is a type of coping skill; it is explained during a cognitive behavioral intervention before LEP stimulation and is employed to distract and draw attention away from the pain symptoms (27-29).
Electrophysiological assessment
During each session, the patients were seated on a couch in a warm, semi-darkened room. LEPs (Nd-YAP, ELEN, Florence, Italy) were evoked by cutaneous heat stimuli on the dorsum of the dominant hand (stimulation parameters: 5-mm beam diameter, 5-msec pulse duration, 1.4-µm wave length, stimulus intensity at pain threshold equivalent to a maximum energy of 6 joule). The site of laser impact was visualized with a He-Ne laser beam and was changed slightly between two successive stimuli to avoid nociceptor sensitization. The pain threshold was calculated using the limits method: a stimulus was delivered by increasing its energy until reaching the pain threshold. Painful stimuli were rated on an 11-point NRS (the pain threshold was defined as a laser stimulus intensity corresponding to point 4 on the NRS). This stimulus intensity was then maintained constant during the test.
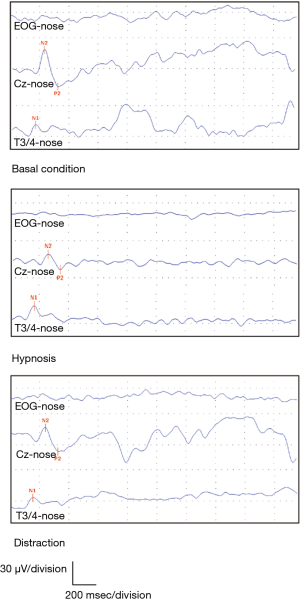
A total of 25–30 laser stimuli were delivered in each trial, and data analysis was made off-line. LEPs were recorded (Keypoint EMG equipment, Dantec, Skovlunde, Denmark) with surface Ag-AgCl electrodes positioned at Cz, Fz, T3/T4, with reference to the nose, according to the 10–20 International System; ocular movement was monitored by electro-oculography (EOG), and EOG-contaminated sweeps were rejected by visual inspection. The electroencephalographic (EEG) signal was amplified and filtered (30–50 µVolt/division, low filter 0.2 Hz, high filter 100 Hz).
LEP components were identified by their latency and polarity and labelled according to Valeriani and co-workers (30). The vertex N2-P2 complex and lateralized temporal N1 latency and amplitude were measured. The procedure was repeated in three different sessions:
- Phase I or basal condition (BC): LEPs were recorded on the dominant hand during the resting state. The patients were asked to mentally count the number of perceived laser stimuli to maintain their attention level constant during the session;
- Phase II or hypnosis condition (HC): LEPs were recorded (on the same hand) while the patients received hypnosis by an expert psychotherapist. They were given relaxation imagery (i.e., walking on a beach) and suggestions for analgesia (for details, see hypnotic induction methods). LEPs were recorded during the entire hypnosis induction, which lasted about 20 minutes;
- Phase III or distraction of attention condition (DC): LEPs were recorded while the patients listened to the reading aloud of a content-neutral magazine article especially selected to avoid evoking emotions or reactions. In this session, performed at least 3 hours after hypnotic induction, the patients were instructed to remember as much as possible about the article.
At the end of each session, the patients were asked to quantify on the NRS the intensity of the perceived pain elicited by LEP stimulation and its unpleasantness. They were asked whether their chronic pain had been present at the beginning of the experiment and whether it changed during hypnotic induction and after listening to the article reading.
The study was approved by the local Ethical Committee of Verona University. Written informed consent was obtained from all patients.
Statistical analysis
LEP amplitudes and latencies are expressed as mean ± standard deviation (SD) as they showed Gaussian distribution. To evaluate the interference of hypnosis and DA on LEP measures, N1, N2-P2 amplitudes, latencies and NRS pain ratings in the three conditions (phase I, II, III) were compared using one-way analysis of variance (ANOVA) for repeated measures, where the condition (BC, HC, and DC) was the within-subject factor. Bonferroni’s correction was applied for post hoc analysis.
We calculated an N2-P2 ratio, which represents the N2-P2 amplitude rate between HC and BC (N2-P2 HC/BC) and between DC and BC (N2-P2 DC/BC) to express the reduction in N2-P2 amplitude as a variable of interest. The two N2-P2 ratios were then compared using an unpaired Student’s t-test. Moreover, to evaluate the relationship between electrophysiological variables, NRS scores for pain intensity and unpleasantness during hypnosis, Pearson’s correlation coefficient between the LEP amplitudes and the corresponding NRS values was calculated. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences, IBM-SPSS, Armonk, NY, USA). The significance level was set at P≤0.05.
Results
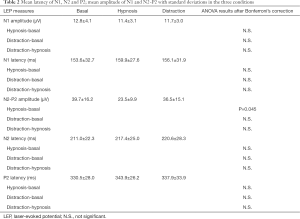
The mean latency of N1, N2, P2 and N1, N2-P2 amplitude during the three conditions are presented in Table 2; the mean NRS scores are reported in Table 3.
Full table

Full table
No changes were observed in the N1 amplitude or N2, P2, and N1 latencies in the three conditions (Figure 1). Conversely, a statistically significant difference in N2-P2 amplitudes was found between HC and BC, (F=3.78, P=0.045) but not between HC and DC or between DC and BC (Figure 2A, Table 2).
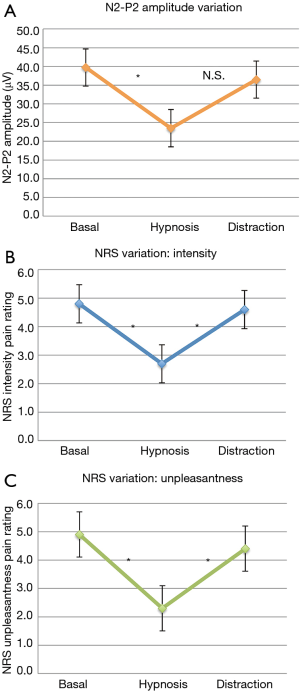
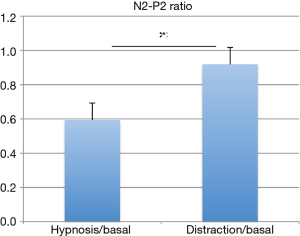
We observed a statistically significant difference in ratings of pain intensity and unpleasantness between HC and BC (F=7.5, P=0.005 for intensity, and F=13.74, P<0.0001 for unpleasantness) and between HC and DC (F=7.5, P=0.010 for intensity, and F=13.74, P=0.001 for unpleasantness). No significant differences in mean laser pain ratings were observed for NRS scores between DC and BC (Figure 2B,C). The N2-P2 amplitude rate, expressing the N2-P2 amplitude ratio between HC and DC versus BC, showed a marked, significant reduction during HC (N2-P2 HC/BC ratio, 0.59±0.07) when compared to DC (N2-P2 DC/BC ratio, 0.92±0.05) (P<0.0001) (Figure 3).
Pearson’s correlation coefficient between N2-P2 amplitudes and NRS scores of pain intensity and unpleasantness showed a statistically significant correlation between the decrease in N2-P2 during hypnosis and in NRS scores for laser-induced pain (r=0.5, P=0.004 for pain intensity; r=0.38, P=0.036 for pain unpleasantness).
Discussion
Our data demonstrate that hypnosis may significantly reduce pain, as measured by LEP experiments, and LEP N2-P2 complex amplitudes as compared with the control condition (CC) and DA. These findings are shared by previous studies on LEPs and modulation of brain evoked potentials under hypnotic analgesia (31). Arendt-Nielsen et al. (32) found that, in highly hypnotically susceptible subjects, thresholds were reduced and increased, respectively, during induced hyperaesthesia and analgesia. Moreover, the amplitude of the brain evoked potentials was increased during hyperaesthesia and decreased during analgesia. Valentini and co-workers (33) studied whether hypnotic suggestion of sensory and affective hypoalgesia (down condition) or hyperalgesia (up condition) differentially influenced subjective ratings of laser-induced pain and nociceptive-related brain activity in high and low hypnotically suggestible individuals. They found a significant hypnotic modulation of pain intensity and unpleasantness in highly suggestible patients and P2 modulation in the up and down conditions, suggesting a top-down modulatory effect on both evoked and induced cortical brain responses triggered by selective nociceptive laser inputs. These studies provide evidence for the higher efficacy of hypnotic analgesia in highly hypnotizable subjects. Del Percio and colleagues (17) demonstrated, by painful and non-painful electrical stimuli and cortical recording, that the amplitude of the cortical N1-P2 complex was lower in the high-hypnotizability than in the low-hypnotizability group for non-painful and painful stimuli, suggesting a more evident reduction in cortical activity in highly susceptible subjects. Taken together, these findings indicate that “high hypnotizables” may possess an enhanced ability to generate focused attention (or disattention) to information and activity controlled by the so-called pain matrix cerebral areas. Nonetheless, criticism has been raised about the concept of hypnotizability and related scales constructed 50 years ago, in which hypnosis was defined mainly by suggestibility. Though it once played a central role in the past, suggestibility does not appear to be specific to hypnosis (34). Furthermore, it is not clear whether hypnotic capacity is related to suggestibility or, rather, to the subject’s trust, motivation, and cognitive flexibility, since they may lead one to effectively follow the same hypnotic instructions for very different reasons (35).
In the present study, the reduction in the N2-P2 complex after hypnotic induction may have resulted from modulation of pain matrix activity, particularly of the ACC, i.e., the brain area that plays a primary role in generating the vertex complex. Few studies on LEPs and brain-evoked potentials have reported no modification during hypnosis (36,37), though subjective pain reports were significantly decreased under the hypnotic procedure. We can speculate that these discrepancies may be due to technical factors, such as the type of stimulus (superficial or intracutaneous, electrical or laser radiant heat stimulation) (36) or the effect of dissociation between sensorial and cognitive stimuli processing, probably caused by the strong influence of hypnosis on the subjective and affective components of nociception (37). It is also presumable that hypnotic analgesia subtends different cognitive processes and brain mechanisms.
Positron emission tomography (PET) studies investigating the effects of hypnotic analgesia on brain areas and underlying neurophysiological processes have demonstrated that the specific action of hypnotic analgesia on brain areas seems to depend on the type of hypnotic suggestions. For instance, suggestions to reduce the unpleasantness of pain seem to be associated with reduced activity in the ACC (a structure in the limbic system thought to be responsible for processing affective responses to pain) but not in the sensory cortices (structures associated with processing the sensory components of pain, such as intensity) (13). By contrast, hypnotic suggestions to reduce pain intensity resulted in decreased activity in the sensory cortices but not in the ACC (14). While the majority of neuroimaging studies found that hypnotic analgesia can reduce activity in virtually all supraspinal areas subtending the pain matrix (thalamus, sensory cortices, insula, ACC, and frontal attentional networks) (12), a minority found the opposite effect (9). Many other factors besides suggestion can account for such discrepancy, including methodological differences in susceptibility, induction procedure, and suggestion content (38). Therefore, it seems to make sense to talk about hypnotic analgesia in terms of multiple mechanisms, which may justify the different neuroimaging and neurophysiological variables in hypnosis.
We observed a significant reduction in pain intensity and unpleasantness scores in all patients after hypnosis: these observations strengthen the idea that both pain intensity and unpleasantness may be reduced in the hypnotic state, as confirmed by PET studies (15,39). The effect may be due to modulation of different brain areas such as the posterior cingulate cortex (for intensity) and the ACC (for unpleasantness) (40). Furthermore, we recorded a trend approaching a decrease in N2-P2 amplitude in DC vs. BC, albeit without statistical significance (Table 2).
LEP amplitude may be modulated by vigilance or arousal (41). Studies using LEPs to evaluate the power of DA as a method of pain control demonstrated a significant pain reduction when subjects were distracted from noxious stimulation and a significant reduction in LEP amplitude and pain in distraction conditions as compared to CC. In contrast, the subjects displayed enhanced event-related potential amplitudes when they were asked to focus their attention on noxious input (42).
It has been hypothesized that hypnosis and DA might share similar mechanisms and that hypnosis simply represents an extensive state of sustained attention or distraction. Based on this assumption, Friederich and collaborators (37) recorded event-related electrical brain potentials to noxious laser-heat stimuli and pain reports during hypnosis analgesia (HA), DA, and CC from highly susceptible subjects. While pain reports were significantly reduced during HA and DA (as compared to CC), LEPs were also significantly smaller during DA but not during HA with respect to CC. They concluded that HA and DA represent different mechanisms of pain control, possibly involving different brain mechanisms.
Also, Freeman and collaborators (43), by demonstrating that highly hypnotizable subjects showed significantly greater pain relief during hypnosis than DA, failed to provide evidence supporting the sociocognitive theory (based on the concept that hypnosis is a sort of attention distraction); instead, they advocated the neo-dissociation and state-based theories of hypnosis.
As regards the extreme variability in brain activation areas during the hypnotic condition, it is plausible that attention, in its function of alerting, orienting, and executive control (7), could subtend different brain activation mechanisms also in pain control. In our pilot study, no significant reduction in pain reports or LEP amplitude after DA was noted. Despite the close links between hypnosis and attention networks, our findings suggest that hypnosis is a complex phenomenon that probably implicates different brain functions besides attentional processes. Furthermore, unlike the change in N2-P2 after modulation under hypnosis, the lateralized early component, or N1 wave, remained unchanged across all three sessions. The lateralized N1 component is less affected by attentive and distractive conditions (42) because it reflects a different function (sensory aspects) than the late N2-P2 complex (emotional and subjective pain experience) in pain processing.
Our study subjects were familiar with hypnosis. Importantly, we did not intentionally select the most highly suggestible patients because the study purpose was to compare neurophysiological parameters during hypnosis vs. DA and not to differentiate high from low hypnotizable subjects. Our findings were not influenced by the so-called habituation phenomenon (characterized by a progressive decrease in amplitude across successive stimuli, with larger responses occurring at the beginning and smaller responses at the end of stimulation), since the hypnotic session preceded the DA session, where the effect of habituation on LEP amplitudes would be more evident at the end of stimulation.
Conclusions
Our findings support the hypothesis that hypnosis inhibits afferent nociceptive transmission; the physiological mechanism of hypnosis may involve sub-cortical gating processes on cortical activation that underlies decreased subjective pain perception and LEP modulation reported by subjects under hypnosis. Taken together, these findings indicate that clinical hypnosis can play a key role in maximizing both behavioral and neurophysiological responses since hypnosis is a cognitive phenomenon that affects central nociceptive processing. Although hypnosis has been used for at least as long as recorded history, we are only now beginning to understand its neurophysiological foundations (44). Future research with larger samples is needed to explore how clinical hypnosis can modulate neurophysiological processes within the brain and measure its effectiveness on pain relief.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Ethical Committee of Verona University. Written informed consent was obtained from all patients for publication of this manuscript and any accompanying images.
References
- Elkins GR, Barabasz AF, Council JR, et al. Advancing Research and Practice: The Revised APA Division 30 Definition of Hypnosis. Am J Clin Hypn 2015;57:378-85. [Crossref] [PubMed]
- Landry M, Appourchaux K, Raz A. Elucidating unconscious processing with instrumental hypnosis. Front Psychol 2014;5:785. [Crossref] [PubMed]
- Tashjian VC, Mosadeghi S, Howard AR, et al. Virtual Reality for Management of Pain in Hospitalized Patients: Results of a Controlled Trial. JMIR Ment Health 2017;4:e9. [Crossref] [PubMed]
- McGeown WJ, Mazzoni G, Venneri A, et al. Hypnotic induction decreases anterior default mode activity. Conscious Cogn 2009;18:848-55. [Crossref] [PubMed]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci 2005;8:1784-90. [Crossref] [PubMed]
- Raz A, Fan J, Posner MI. Hypnotic suggestion reduces conflict in the human brain. Proc Natl Acad Sci U S A 2005;102:9978-83. [Crossref] [PubMed]
- Raz A, Fan J, Posner MI. Neuroimaging and genetic associations of attentional and hypnotic processes. J Physiol Paris 2006;99:483-91. [Crossref] [PubMed]
- Raz A, Campbell N, Guindi D, et al. Placebos in clinical practice: comparing attitudes, beliefs, and patterns of use between academic psychiatrists and nonpsychiatrists. Can J Psychiatry 2011;56:198-208. [Crossref] [PubMed]
- Landry M, Lifshitz M, Raz A. Brain correlates of hypnosis: A systematic review and meta-analytic exploration. Neurosci Biobehav Rev. 2017;81:75-98. [Crossref] [PubMed]
- Terhune DB, Cleeremans A, Raz A, et al. Hypnosis and top-down regulation of consciousness. Neurosci Biobehav Rev. 2017;81:59-74. [Crossref] [PubMed]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci 2006;7:367-79. [Crossref] [PubMed]
- Jensen MP, Patterson DR. Hypnotic approaches for chronic pain management: clinical implications of recent research findings. Am Psychol 2014;69:167-77. [Crossref] [PubMed]
- Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968-71. [Crossref] [PubMed]
- Hofbauer RK, Rainville P, Duncan GH, et al. Cortical representation of the sensory dimension of pain. J Neurophysiol 2001;86:402-11. [Crossref] [PubMed]
- Faymonville ME, Laureys S, Degueldre C, et al. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology 2000;92:1257-67. [Crossref] [PubMed]
- Fingelkurts AA, Fingelkurts AA, Kallio S, et al. Cortex functional connectivity as a neurophysiological correlate of hypnosis: an EEG case study. Neuropsychologia 2007;45:1452-62. [Crossref] [PubMed]
- Del Percio C, Triggiani AI, Marzano N, et al. Subjects' hypnotizability level affects somatosensory evoked potentials to non-painful and painful stimuli. Clin Neurophysiol 2013;124:1448-55. [Crossref] [PubMed]
- Derbyshire SW, Whalley MG, Stenger VA, et al. Cerebral activation during hypnotically induced and imagined pain. Neuroimage 2004;23:392-401. [Crossref] [PubMed]
- Stoelb BL, Molton IR, Jensen MP, et al. The efficacy of hypnotic analgesia in adults: a review of the literature. Contemp Hypn 2009;26:24-39. [Crossref] [PubMed]
- Jensen MP. Hypnosis for chronic pain management: a new hope. Pain 2009;146:235-7. [Crossref] [PubMed]
- Brugnoli MP. Clinical hypnosis in pain therapy and palliative care: a handbook of techniques for improving the patient's physical and psychological well-being. Springfield, Illinois, U.S.A.: Charles C Thomas Publisher, Ltd., 2014. ISBN 9780398087654.
- Brugnoli MP. Clinical hypnosis for palliative care in severe chronic diseases: a review and the procedures for relieving physical, psychological and spiritual symptoms. Ann Palliat Med 2016;5:280-97. [Crossref] [PubMed]
- Vanhaudenhuyse A, Laureys S, Faymonville ME. Neurophysiology of hypnosis. Neurophysiol Clin 2014;44:343-53. [Crossref] [PubMed]
- Tefikow S, Barth J, Maichrowitz S, et al. Efficacy of hypnosis in adults undergoing surgery or medical procedures: a meta-analysis of randomized controlled trials. Clin Psychol Rev 2013;33:623-36. [Crossref] [PubMed]
- Elkins G, Jensen MP, Patterson DR. Hypnotherapy for the management of chronic pain. Int J Clin Exp Hypn 2007;55:275-87. [Crossref] [PubMed]
- Green JP, Barabasz AF, Barrett D, et al. Forging ahead: the 2003 APA Division 30 definition of hypnosis. Int J Clin Exp Hypn 2005;53:259-64. [Crossref] [PubMed]
- Abdelmoniem SA, Mahmoud SA. Comparative evaluation of passive, active, and passive-active distraction techniques on pain perception during local anesthesia administration in children. J Adv Res 2016;7:551-6. [Crossref] [PubMed]
- Masuda A, Feinstein AB, Wendell JW, et al. Cognitive defusion versus thought distraction: a clinical rationale, training, and experiential exercise in altering psychological impacts of negative self-referential thoughts. Behav Modif 2010;34:520-38. [Crossref] [PubMed]
- Cocude M, Denis M. Measuring the temporal characteristics of visual images. J Ment Imag 1988;12:89-101.
- Valeriani M, Rambaud L, Mauguière F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand. Electroenceph Clin Neurophysiol 1996;100:343-53. [Crossref] [PubMed]
- Zachariae R, Bjerring P. Laser-induced pain-related brain potentials and sensory pain ratings in high and low hypnotizable subjects during hypnotic suggestions of relaxation, dissociated imagery, focused analgesia, and placebo. Int J Clin Exp Hypn 1994;42:56-80. [Crossref] [PubMed]
- Arendt-Nielsen L, Zachariae R, Bjerring P. Quantitative evaluation of hypnotically suggested hyperaesthesia and analgesia by painful laser stimulation. Pain 1990;42:243-51. [Crossref] [PubMed]
- Valentini E, Betti V, Hu L, et al. Hypnotic modulation of pain perception and of brain activity triggered by nociceptive laser stimuli. Cortex 2013;49:446-62. [Crossref] [PubMed]
- Tasso AF, Pérez NA. Parsing everyday suggestibility: What does it tells us about hypnosis? In Nash MR, Barnier AJ. editors. The Oxford handbook of hypnosis. New York, NY: Oxford University Press, 2008:283-309.
- Lifshitz M, Howells C, Raz A. Can expectation enhance response to suggestion? De-automatization illuminates a conundrum. Conscious Cogn 2012;21:1001-8. [Crossref] [PubMed]
- Meier W, Klucken M, Soyka D, et al. Hypnotic hypo- and hyperalgesia: divergent effects on pain ratings and pain-related cerebral potentials. Pain 1993;53:175-81. [Crossref] [PubMed]
- Friederich M, Trippe RH, Ozcan M, et al. Laser-evoked potentials to noxious stimulation during hypnotic analgesia and distraction of attention suggest different brain mechanisms of pain control. Psychophysiology 2001;38:768-76. [Crossref] [PubMed]
- Landry M, Raz A. Hypnosis and imaging of the living human brain. Am J Clin Hypn 2015;57:285-313. [Crossref] [PubMed]
- Del Casale A, Ferracuti S, Rapinesi C, et al. Pain perception and hypnosis: findings from recent functional neuroimaging studies. Int J Clin Exp Hypn 2015;63:144-70. [Crossref] [PubMed]
- Tölle TR, Kaufmann T, Siessmeier T, et al. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol 1999;45:40-7. [Crossref] [PubMed]
- Beydoun A, Morrow TJ, Shen JF, et al. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol 1993;88:173-81. [Crossref] [PubMed]
- García-Larrea L, Peyron R, Laurent B, et al. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport 1997;8:3785-9. [Crossref] [PubMed]
- Freeman R, Barabasz A, Barabasz M, et al. Hypnosis and distraction differ in their effects on cold pressor pain. Am J Clin Hypn 2000;43:137-48. [Crossref] [PubMed]
- Jensen MP, Adachi T, Hakimian S. Brain Oscillations, Hypnosis, and Hypnotizability. Am J Clin Hypn 2015;57:230-53. [Crossref]


