Prognostic indicators of neuromuscular disorders for palliative care referral
Introduction
Neuromuscular disorders (NMDs) refer to diseases that affect the nervous system from the lower motor neuron to the muscle. They can involve the anterior horn cell, for example the motor neuron disease (MND); the myelin and axon of the neuron, as exemplified by various neuropathies; the neuromuscular junction, such as the myasthenia gravis; and the muscle, namely both acquired and congenital myopathies. Some of them are curable or controllable by specific therapies, hence projecting favorable outcomes. However, a large portion has no effective treatment and run an intractable course, having grave prognosis. These cases, including MND and muscular dystrophies, could be ideal candidates for palliative care (1). Symptom relief, psychosocial support and end-of life planning are needed. Despite that, the timing of palliative service referral is uncertain (2). Few studies have been done to investigate the trajectories of incurable NMDs in palliative medicine perspective. This study is aimed to investigate various symptoms and their predictive values in early mortality. It is hope to help clinicians to identify NMD cases that need early referral to palliative service.
Methods
Study design and data collection
A single center and retrospective study was conducted. The computerized database of a university hospital in Hong Kong was searched to identify patients with diagnostic labels of NMD in the period of 2009 to 2016. The diagnosis should be made at adult age of 18-year-old or above. Demographic data and clinical profiles were extracted.
Data analysis
Outcome was defined as early death from any causes within the first year after the NMD diagnosis. Early-onset of a symptom was defined as the presentation of the symptom within the first year after the NMD diagnosis. Impaired mobility was defined as modified ranking scale of 4 or below. Age, gender and early-onset symptoms were correlated with the outcome using Pearson’s correlation coefficient. Prediction models for early death were formulated and their performance was assessed. Chi-square test and binary logistic regression were employed for analysis. Area under the receiver operating characteristic (ROC) curve was used to test the model performance. Statistical analysis was performed with SPSS version 24. Statistical significance was defined as P<0.05.
Results
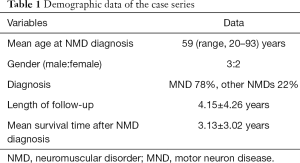
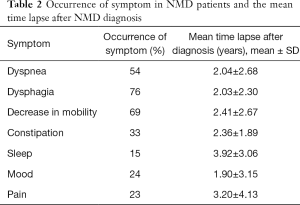
Total of 86 patients were recruited for analysis. There were 67 MND cases (78%) (Table 1). Other myopathies accounted for 19 cases (22%). They included mitochondrial myopathies, myotonia dystrophica, facioscapulohumeral muscular dystrophy, etc. Mean age at NMD diagnosis was 59 years (range, 20–93 years). Male to female ratio was about 3:2. The mean follow-up duration was 4.15±4.26 years. Mean survival time was 3.13±3.02 years after diagnosis. Different symptoms were documented (Table 2).
Full table
Full table
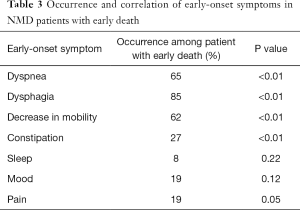
Variables of gender, age of diagnosis and early-onset symptoms were correlated with early death individually by t-test and Chi’s square (or Fisher’s exact test) (Table 3). NMD onset age, early-onset dyspnea, dysphagia, impaired mobility and constipation were found to have significant correlation with early death.
Full table
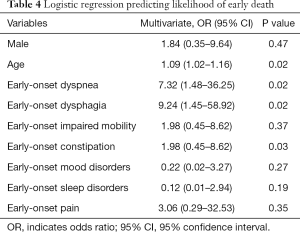
Logistic regression model was used to investigate the effects of early-onset dyspnea, dysphagia, impaired mobility, constipation, mood, sleep disorders and pain on the likelihood of early death. Male and age at diagnosis were also included in the analysis. Linearity of the continuous variable with respect to the logit of the dependent variable was verified by the Box-Tidwell procedure with application of Bonferroni correction. Age was linearly related to the logit of the dependent variable. The logistic regression model was statistically significant, χ2[9] =54.08, P<0.001. The model explained 66.1% (Nagelkerke R2) of the variance in the outcome and correctly classified 85% of cases. Sensitivity was 76.92%, specificity was 88.33%, positive predictive value was 74.07% and negative predictive value was 89.83%. Of the nine variables, four were statistically significant: age of NMD onset, early-onset dyspnea, dysphagia and constipation (Table 4).
Full table
Three models were established, trying to associate different variable combinations with the outcome of earth death (Table 5). Area under ROC curve was used to assess the accuracy of prediction. Model 3, which includes variables of age on NMD onset, early onsets of dyspnea, dysphagia, constipation and impaired mobility, has the best performance in predicting early death within one year of NMD diagnosis.

Full table
Discussion
The results show the age of NMD diagnosis and early emergence of certain symptoms are associated with early death. They may serve as predictors of early mortality and hence indicators of palliative care referral in this group of patients. This is consistent with the previous study findings (3). Age itself is correlated with life expectancy and also related to general morbidity state. Bulbar onset MND has been found to have poorer prognosis (4). Dysphagia can directly threaten a patient’s life by choking with suffocation and aspiration pneumonia. Also, dysphagia can result in malnutrition (5,6). Dyspnea is related to poor respiratory muscles and weakened diaphragm (7,8). In fact, the emergence of dyspnea may signify the final state of prolonged respiratory failure (9). Neuromuscular diseases typically complicated by type II respiratory failure with carbon dioxide retention. Constipation has multifactorial causes, such as autonomic failure, immobility and side effects of medications (10-12). Importantly, it may reflect poor abdominal and diaphragmatic muscles (13). This symptom may be easily overlooked. Attention should be paid to this symptom as it may predict early mortality especially if it is presented early in the course of disease. Mobility depends on limb muscle power and so as the respiratory function. Although early-onset pain, sleep and mood disorders are not found to have correlations with short-term mortality, these symptoms are could be ignored. Mood and sleep symptoms are not uncommon and have great implication in the patient’s quality of life (14-16). Despite, motor system is predominantly involved in NMDs, pain and other sensory symptoms are common in this patient group (17,18). These symptoms could be major treatment goals of palliative care.
There are several limitations in the study. This was a retrospective study with recall bias. The study group mainly consisted of patients with MND. MND has faster progression than other NMDs in general. Symptom occurrence depended on reporting rather than systemic questioning and may cause reporting bias. Symptoms were only interpreted as either presence or absence but not graded in established severity scores.
Conclusions
This study describes the symptomatology of NMDs which may serve as triggers for palliative care. It shows that onset age and certain early-onset symptoms including dyspnea, dysphagia, impaired motility and constipation could predict early mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethical approval is by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (No. UW-16-187). This was a retrospective study, all subjects were anonymous, written consent was not applicable.
References
- Carter GT, Joyce NC, Abresch AL, et al. Using palliative care in progressive neuromuscular disease to maximize quality of life. Phys Med Rehabil Clin N Am 2012;23:903-9. [Crossref] [PubMed]
- Tripodoro VA, De Vito EL. What does end stage in neuromuscular diseases mean? Key approach-based transitions. Curr Opin Support Palliat Care 2015;9:361-8. [Crossref] [PubMed]
- Lunetta C, Lizio A, Melazzini MG, et al. Amyotrophic Lateral Sclerosis Survival Score (ALS-SS): A simple scoring system for early prediction of patient survival. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:93-100. [Crossref] [PubMed]
- Turner MR, Scaber J, Goodfellow JA, et al. The diagnostic pathway and prognosis in bulbar-onset amyotrophic lateral sclerosis. J Neurol Sci 2010;294:81-5. [Crossref] [PubMed]
- Toussaint M, Davidson Z, Bouvoie V, et al. Dysphagia in Duchenne muscular dystrophy: practical recommendations to guide management. Disabil Rehabil 2016;38:2052-62. [Crossref] [PubMed]
- Willig TN, Paulus J, Lacau Saint Guily J, et al. Swallowing problems in neuromuscular disorders. Arch Phys Med Rehabil 1994;75:1175-81. [Crossref] [PubMed]
- Quijano-Roy S, Khirani S, Colella M, et al. Diaphragmatic dysfunction in Collagen VI myopathies. Neuromuscul Disord 2014;24:125-33. [Crossref] [PubMed]
- Simon NG, Kiernan MC. Diaphragm ultrasound in amyotrophic lateral sclerosis and other neuromuscular disorders. Clin Neurophysiol 2016;127:28-30. [Crossref] [PubMed]
- Howard RS, Wiles CM, Hirsch NP, et al. Respiratory involvement in primary muscle disorders: assessment and management. Q J Med 1993;86:175-89. [PubMed]
- Jackson CE, McVey AL, Rudnicki S, et al. Symptom Management and End-of-Life Care in Amyotrophic Lateral Sclerosis. Neurol Clin 2015;33:889-908. [Crossref] [PubMed]
- Piccione EA, Sletten DM, Staff NP, et al. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve 2015;51:676-9. [Crossref] [PubMed]
- Lo Cascio CM, Goetze O, Latshang TD, et al. Gastrointestinal Dysfunction in Patients with Duchenne Muscular Dystrophy. PLoS One 2016;11. [Crossref] [PubMed]
- Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci 2012;57:1445-64. [Crossref] [PubMed]
- Graham CD, Weinman J, Sadjadi R, et al. A multicentre postal survey investigating the contribution of illness perceptions, coping and optimism to quality of life and mood in adults with muscle disease. Clin Rehabil 2014;28:508-19. [Crossref] [PubMed]
- Rose MR, Sadjadi R, Weinman J, et al. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve 2012;46:351-9. [Crossref] [PubMed]
- Laberge L, Bégin P, Dauvilliers Y, et al. A polysomnographic study of daytime sleepiness in myotonic dystrophy type 1. J Neurol Neurosurg Psychiatry 2009;80:642-6. [Crossref] [PubMed]
- Chiò A, Mora G, Lauria G. Pain in amyotrophic lateral sclerosis. Lancet Neurol 2017;16:144-57. [Crossref] [PubMed]
- Lager C, Kroksmark AK. Pain in adolescents with spinal muscular atrophy and Duchenne and Becker muscular dystrophy. Eur J Paediatr Neurol 2015;19:537-46. [Crossref] [PubMed]


