Does gender affect self-perceived pain in cancer patients? —A meta-analysis
Introduction
Improved medical advances have resulted in a greater life expectancy and growing cancer incidence in the global population (1,2). Pain is present in 50–70% of cancer patients and is amongst the most feared symptom of cancer despite improved analgesic efficiency and decreased mortality (3-5).
A review by Fillingim et al. demonstrates a considerable interest in the investigation of gender differences in pain since the 1990s (6). Past clinical knowledge and speculation has led to the conclusion that females have an increased likelihood of experiencing pain compared to males, which has been corroborated by past research. The majority of studies have reported a female inclination towards greater pain sensitivity, while other articles find non-significant gender differences in pain intensity (6). Overall, pain seems to manifest more favourably in females compared to males as outlined in reviews conducted by Fillingim and Vallerand (6,7).
There has been speculation regarding the cause for previously reported gender differences in pain. Increased investigation has caused many researchers to seek reasoning behind possible gender differences in pain perception. First, physiologic differences exist: for example, ovarian hormone may be responsible for increased serotonergic activity, thus rendering females more susceptible to chronic pain disorders (6). Physiological differences between males and females support the possibility that cancer may uniquely impact pain perception in different sexes. Females may also exhibit further sequelae commonly associated with pain, such as anxiety. A final reason related to the increased frequency of comorbidities in females, which may enhance self-perceived pain (8).
Gender stereotypes have been thought to mediate self-reported pain scores via psychosocial mechanisms (6). A study by Otto revealed greater masculinity and femininity to be associated with higher and lower pain thresholds, respectively (9). Evidence also suggests that women consider men less likely to report pain while men rate women as more sensitive (10,11). Psychosocial factors should be considered in explaining observable gender differences in pain perception.
There is a strong association of pain with cancer. Since pain is a primary concern among cancer patients, examination of gender differences in pain experience may improve clinical decision-making and management related to cancer-induced pain (4). For example, possible differences in baseline cancer pain intensity by gender may have implications for the dosing regimens of analgesics. Despite the need to examine the relationship between gender and pain, research in the cancer setting is under-reported and reveals conflicting evidence (12).
This meta-analysis aimed to synthesize all comparative literature estimates concerning gender differences in self-perceived pain in cancer.
Methods
Search strategy
A literature search was conducted through Ovid MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials [1947–2016] to identify studies examining gender differences in self-perceived pain in cancer patients. Keywords included “pain”, “pain management”, “analgesia”, “sex factor”, “sex difference”, “gender,” “sex”, “sexes”, “male”, “female”, “men”, “women”, “neoplasms”, “cancer”, and “tumour”. Reference lists of included studies were also examined for relevant literature. The search strategies for respective databases are outlined in Appendix 1.
Studies which examined less than five patients (n<5), were not written in English, or used performance/quality of life (QOL) questionnaires to measure pain were excluded. Only studies reporting baseline pain scores as a continuous variable specific to each gender were included. Clinical trials that utilized specific pain measurement tools with 0–10 or 0–100 scales were included. Pain severity scores from the Numerical Rating Scale (NRS), Visual Analogue Scale (VAS), European Symptom Assessment Scale (ESAS), and the Brief Pain Inventory (BPI) questionnaires for pain were chosen for analysis. Subgroup analyses stratified by pain scale and cancer severity were performed to compare data from similarly designed studies.
Pain measurement tools
VAS
The VAS is a self-administered tool used to measure the intensity of symptoms such as nausea, anxiety, and pain. The VAS pain scale consists of a 100-mm line where the start and end of the line corresponds with “no pain” and “worst imaginable pain”, respectively (13). Patients are instructed to point to a location on the 100-mm line which represents their pain intensity at a specific time interval (e.g., over past 24 hours). In the cancer setting, studies have reported good reliability in measuring pain using the VAS (14). The VAS involves minimal administrative burden, and studies have reported good test reliability in literate patients (13). However, conceptual difficulty in visualizing line-scale representation of pain is a concern especially amongst elderly patients and those with limited literacy (13,14).
NRS
Similar to the VAS tool, NRS is often used to assess general symptoms including pain severity. Variations of the NRS include 6-point [0–5], 11-point [0–10], and 101-point [0–100] scales with a general consensus that the 11-point scale provides the most ideal user-interface while providing sufficient informative data (15). Patients are instructed to select a number on a numbered scale which best represents one’s symptom severity. The NRS tool displays high test reliability in all patients independent of literacy (13). Studies using the 0–10 NRS have shown high correlation to VAS scores (13), while discrepancies in translation of pain scores between 6-point NRS and 100-mm VAS tools have been reported (16). For the purpose of this meta-analysis, VAS and 11-point NRS scores were grouped together for meta-analysis.
ESAS
The ESAS is a self-reporting tool used to measure symptom intensity in advanced cancer patients. The ESAS comprises nine symptoms: pain, tiredness, drowsiness, nausea, appetite, shortness of breath, depression, anxiety, and well-being. Each symptom is rated on an 11-point NRS scale (17) over a specific time interval, such as “over the past 24 hours”. This tool is frequently used in palliative clinics due to its reliability, validity (18) and ease of use (19). For this reason, studies which utilized the ESAS for pain were classified in the same category as those reporting on the NRS.
BPI
The BPI is a self-administered questionnaire that measures two components of pain: (I) pain severity and (II) pain interference (i.e., the extent of how pain hinders daily function). Both measures are quantified on an 11-point 0–10 scale. Each aspect is calculated by averaging results from four 0–10 scales within the BPI (14). The BPI has been translated in multiple languages and uses a 24-hour recall period. The long-form questionnaire includes additional questions on patient demographics and pain history, and is thus more often utilized for baseline pain measures. In contrast, the brevity of the short-form questionnaire makes it appealing for repetitive tasks and research purposes (20). Pain severity measurements include intensity scores at “least”, “worst”, “average” and “present” timeframes. The BPI pain form demonstrates good reliability and validity (21,22).
Data collection and analysis strategy
The following data were collected from included studies using Microsoft Excel: authorship, year of publication, country of origin, age at baseline, gender, history of chemotherapy, radiotherapy or surgery, education, pain assessment tool used, cancer site, presence of metastases, cancer stage, sample size, and average pain severity scores at baseline (mean ± SD) standardized to an 11-point scale from 0–10. For the purposes of this meta-analysis, only average pain severity scores at the beginning of clinical trial entry were considered.
To quantify differences in self-reported pain severity between gender groups, forest plots were constructed using random-effects modelling with Review Manager 5.3 Cochrane Collaboration software. Results were reported throughout using weighted mean differences and 95% confidence intervals (CI). The total number of patients from each study and/or study arm was considered a weighting variable. Independent meta-analyses were conducted based on pain measurement tool used (BPI and NRS/VAS groups). Subgroup analysis was also conducted based on cancer severity. Using random-effects modeling, weighted mean differences and 95% CI were used to estimate the effect of gender on pain severity in cancer patients. A P value of less than 0.05 was considered statistically significant. This review was produced in accordance with MOOSE guidelines.
Results
Search results and study characteristics
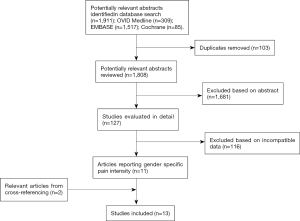
From a literature search of 1,911 articles, title and abstract screening resulted in the preliminary inclusion of 127 studies. After full text screening, a total of 15 study arms from 13 articles met all inclusion criteria and were included into the meta-analysis (Figure 1) (23-35).
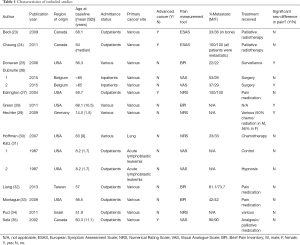
Socio-demographic and clinical characteristics of included studies are outlined in Table 1. Included studies (n=13) contained a total of 2,017 male and 2,256 female cancer patients. The weighted mean age of male patients (64.4±17.0 years) was greater than female patients (59.3±15.3 years) across 8 of the 13 studies (23,25,27-29,32,34,35), however this difference was not significant. A total of 7 study arms were conducted in the US (25,27,28,30,31,33), 3 in Canada (23,24,35), 3 in Europe (26,29), and 2 in Asia (32,34). Twelve of 15 study arms examined a patient cohort with diverse primary cancers (23-29,32-35) while the Katz article (31) and the Hoffman study (30) investigated pain specific to acute lymphoblastoma and lung cancer, respectively. Mean age at baseline generally ranged from 50–60 years old with the exception of two studies, one investigating pain intensity in children (8.2±1.7 years) (31) and another in adolescents (14.0±1.5 years) (29).
Full table
Results: NRS/VAS
Eleven study arms utilized either the NRS or VAS pain scales. Pain ratings were standardized to a 0–10 score. Figure 2 displays mean differences in pain scores between genders. Four arms reported greater pain ratings in males compared to female ratings (26,27,30,35), however these differences were not statistically significant (P>0.05). In the 11 comparisons (n=3,752), the overall weighted mean difference (95% CI) in pain severity was −0.26 (95% CI: −0.57 to 0.04, P=0.09). There were no significant differences between genders in NRS/VAS pain scores.
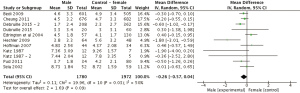
For the subgroup analysis, four study arms within the NRS/VAS group investigated gender differences in pain in advanced cancer patients (Figure 3). Weighted mean difference (95% CI) in pain severity was −0.08 (n=2,762, 95% CI: −0.36 to 0.20, P=0.58). Once again, there were no significant differences in pain scores between comparators.
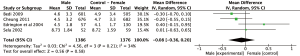
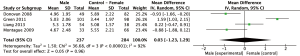
Results: BPI
Meta-analysis of the four studies using the BPI to measure pain severity revealed a non-significant weighted mean difference (95% CI) of 0.03 (n=521, 95% CI: −1.23 to 1.29, P=0.96) as outlined in Figure 4. Observed mean difference in pain intensity was smallest compared to all other analyses sets.
Discussion
This meta-analysis examined the role of gender in pain intensity in cancer patients. Results from meta-analysis demonstrated no significant differences in self-perceived pain between genders in oncology patients.
Gender differences in cancer-related pain have been the subject of several review articles (6,12). A review article conducted by Miaskowski examined four studies, all of which did not report significant gender differences in cancer pain (12). Additionally, Fillingim et al. published a thorough review of the relationship between gender and pain (6). The authors found that the majority of articles investigating gender differences in pain in both the cancer and non-cancer setting typically report either significantly greater pain scores in females or insignificant gender differences in pain (6). Rarely has it been found that men experience significantly more severe pain scores than women (28). These trends were somewhat reflected in our meta-analysis. Across the 15 included study arms, a total of 3 reported significantly greater mean pain severity in females, while 1 showed significantly greater pain scores in males.
A subgroup analysis of gender differences in pain was performed on a group of advanced cancer patients. Chronic pain is reported in 80–90% of patients with metastatic cancer (36). Because pain management is a primary objective with palliative care patients, investigation into gender-specific pain differences in advanced cancer patients is of great relevance. In our advanced cancer subgroup, mean differences in gender pain scores were not significantly different between genders (Figure 3), thus analgesic administration should not be modified based on gender. Male and female patients should be treated equally for management of pain, without assuming that females have greater pain sensitivity. However, given that only four studies have been published in this setting, future investigations should explore gender differences in pain amongst advanced cancer patients. This will facilitate greater confidence in the validity of this observation.
Limitations of this meta-analysis include possible reporting bias in the existing literature. Due to the relative ease of prevalence reporting, a greater proportion of research articles exploring patient pain experience favour the use of prevalence data as opposed to severity measures (6). Pain severity scores in studies investigating cancer patient gender differences are sparse and may not ultimately translate similarly with pain prevalence results. Severity ratings were chosen because they provide a more multi-dimensional measure of pain suitable for meta-analysis.
Certain cancers may cause more pain than others and result in differing severity between genders. For example, somatic pain has been found to be more common in men while women tend to suffer more from visceral pain (37). Our meta-analysis included a wide variety of cancers. Unfortunately, only 3 out of the 15 study arms reported on one type of primary cancer type (30,31) which negated the ability to perform further subgroup analysis by cancer site. As well, it is possible that gender differences in pain may be more pronounced at different stages of cancer (36). While these questions were not able to be explored, researchers should consider them topics of interest for future studies.
Conclusions
In closing, there does not appear to be a significant difference in self-reported cancer pain scores between genders. Future examination into gender differences in pain based on primary cancer type and stage is recommended.
Supplementary
Appendix 1 Search strategies by database
(A) Database: Ovid MEDLINE (R) <1946 to December Week 1 2016>
Search strategy:
1. exp pain/ (369683)
2. exp pain management/ (26750)
3. exp sex factors/ (258241)
4. [(gender or sex or sexes or male or female or men or women) adj2 (difference* or different)].mp. (81916)
5. (1 or 2) and (3 or 4) (7676)
6. exp neoplasms/ (3221266)
7. 5 and 6 [369]
8. limit 7 to (English language and humans) [309]
(B) Database: Embase Classic + Embase <1947 to 2016 Week 52>
Search strategy:
1. exp pain/ (1165273)
2. exp analgesia/ (151167)
3. (pain adj2 management).mp. (29752)
4. exp sex difference/ (336917)
5. sex factor*.mp. (961)
6. [(gender or sex or sexes or male or female of men or women) adj2 (difference* or different)].mp. (376365)
7. (1 or 2 or 3) and (4 or 5 or 6) (17398)
8. exp neoplasm/ (4162889)
9. 7 and 8 (1689)
10. limit 9 to (human and English language) (1517)
(C) Database: EBM Reviews-Cochrane Central Register of Controlled Trials
Search strategy:
1. exp pain/ or pain.mp. (93098)
2. exp pain management/ or pain management.mp. (4156)
3. analgesia.mp. (21881)
4. exp sex factors/ or sex factor*.mp. (5345)
5. [(gender or sex or sexes or male or female or men or women) adj2 (difference* or different)].mp. (7583)
6. (1 or 2 or 3) and (4 or 5) (1281)
7. exp neoplasms/ or (neoplasm* or cancer* or tumor or tumour).mp. (100049)
8. 6 and 7 [93]
9. limit 8 to English language [85]
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Ferrucci L, Giallauria F, Guralnik JM. Epidemiology of aging. Radiol Clin North Am 2008;46:643-52. [Crossref] [PubMed]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49. [Crossref] [PubMed]
- Strang P. Cancer pain--a provoker of emotional, social and existential distress. Acta Oncol 1998;37:641-4. [Crossref] [PubMed]
- Hashim D, Boffetta P, La Vecchia C, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016;27:926-33. [Crossref] [PubMed]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447-85. [Crossref] [PubMed]
- Vallerand AH, Polomano RC. The relationship of gender to pain. Pain Manag Nurs 2000;1:8-15. [Crossref] [PubMed]
- Valeberg BT, Miaskowski C, Hanestad BR, et al. Demographic, clinical, and pain characteristics are associated with average pain severity groups in a sample of oncology outpatients. J Pain 2008;9:873-82. [Crossref] [PubMed]
- Otto MW, Dougher MJ. Sex differences and personality factors in responsivity to pain. Percept Mot Skills 1985;61:383-90. [Crossref] [PubMed]
- Keogh E. Men, masculinity, and pain. Pain 2015;156:2408-12. [Crossref] [PubMed]
- Myers CD, Riley JL 3rd, Robinson ME. Psychosocial contributions to sex-correlated differences in pain. Clin J Pain 2003;19:225-32. [Crossref] [PubMed]
- Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr 2004.139-43. [Crossref] [PubMed]
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240-52. [Crossref] [PubMed]
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain 2003;4:2-21. [Crossref] [PubMed]
- Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs 1997;20:88-93. [Crossref] [PubMed]
- Carpenter JS, Brockopp D. Comparison of patients' ratings and examination of nurses' responses to pain intensity rating scales. Cancer Nurs 1995;18:292-8. [Crossref] [PubMed]
- Watanabe S, Nekolaichuk C, Beaumont C, et al. The Edmonton symptom assessment system--what do patients think? Support Care Cancer 2009;17:675-83. [Crossref] [PubMed]
- Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol 2009;16:55. [PubMed]
- Strömgren AS, Groenvold M, Pedersen L, et al. Symptomatology of cancer patients in palliative care: content validation of self-assessment questionnaires against medical records. Eur J Cancer 2002;38:788-94. [Crossref] [PubMed]
- Cleeland CS. The Brief Pain Inventory: User Guide. Available online: https://www.mdanderson.org/education-and-research/ departments-programs-and-labs/departments-and-divi- sions/symptom-research/symptom-assessment-tools/BPI_ UserGuide.pdf
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38. [PubMed]
- Stanhope J. Brief Pain Inventory review. Occup Med (Lond) 2016;66:496-7. [Crossref] [PubMed]
- Bedi H, Presutti R, Hird A, et al. Gender Difference in the Edmonton Symptom Assessment System (ESAS) Scores in Patients with Advanced Cancer. J Pain Manag 2009;3:63-71.
- Cheung WY, Le LW, Gagliese L, et al. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer 2011;19:417-23. [Crossref] [PubMed]
- Donovan KA, Taliaferro LA, Brock CW, et al. Sex differences in the adequacy of pain management among patients referred to a multidisciplinary cancer pain clinic. J Pain Symptom Manage 2008;36:167-72. [Crossref] [PubMed]
- Dubruille S, Libert Y, Merckaert I, et al. The prevalence and implications of elderly inpatients' desire for a formal psychological help at the start of cancer treatment. Psychooncology 2015;24:294-301. [Crossref] [PubMed]
- Edrington JM, Paul S, Dodd M, et al. No evidence for sex differences in the severity and treatment of cancer pain. J Pain Symptom Manage 2004;28:225-32. [Crossref] [PubMed]
- Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer 2011;117:1994-2003. [Crossref] [PubMed]
- Hechler T, Chalkiadis GA, Hasan C, et al. Sex differences in pain intensity in adolescents suffering from cancer: differences in pain memories? J Pain 2009;10:586-93. [Crossref] [PubMed]
- Hoffman AJ, Given BA, von Eye A, et al. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 2007;34:785-92. [Crossref] [PubMed]
- Katz ER, Kellerman J, Ellenberg L. Hypnosis in the reduction of acute pain and distress in children with cancer. J Pediatr Psychol 1987;12:379-94. [Crossref] [PubMed]
- Liang SY, Wang TJ, Wu SF, et al. Gender differences associated with pain characteristics and treatment in taiwanese oncology outpatients. Asian Pac J Cancer Prev 2013;14:4077-82. [Crossref] [PubMed]
- Montague L, Green CR. Cancer and breakthrough pain's impact on a diverse population. Pain Med 2009;10:549-61. [Crossref] [PubMed]
- Pud D. Gender differences in predicting quality of life in cancer patients with pain. Eur J Oncol Nurs 2011;15:486-91. [Crossref] [PubMed]
- Sela RA, Bruera E, Conner-spady B, et al. Sensory and affective dimensions of advanced cancer pain. Psychooncology 2002;11:23-34. [Crossref] [PubMed]
- Grond S, Zech D, Diefenbach C, et al. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage 1994;9:372-82. [Crossref] [PubMed]
- Mercadante S, Casuccio A, Pumo S, et al. Factors influencing the opioid response in advanced cancer patients with pain followed at home: the effects of age and gender. Support Care Cancer 2000;8:123-30. [Crossref] [PubMed]