Skin toxicity after palliative chemotherapy containing pegylated liposomal doxorubicin for ovarian cancer patients
Background
Anticancer palliative therapy is applied to local advanced or disseminated cancer patients. The main goal of treatment is to prolong patients’ life and moderate the cancer symptoms. Cure is very rare. That is the reason to pay particular attention to minimize side effects. Skin toxicity in the course of anticancer treatment occurs in majority of patients. It can be caused by chemotherapy, targeted therapy, as well as radiotherapy. They may be often a symptom of paraneoplastic syndrome. Skin toxicity may play an important role on treatment modality, in some cases causing the necessity of interruption or discontinuation the treatment. Although skin toxicity is rarely life-threatening it often worsens the patients’ quality of life. Particularly common skin complications are observed during the anti-EGFR therapy in the form of acneiform eruptions, paronychia, xerosis, fissures, hyperpigmentation, alterations in hair growth and telangiectasia (1,2). Extremely specific skin toxicity are observed during treatment with pegylated liposomal doxorubicin (PLD). PLD is an anthracyclines’ derivative with reducing severity of cardiotoxicity, acute infusion-related toxicity, alopecia and myelosuppression (3-5). However mucocutaneous reactions are seen in an increased in frequency. Palmar-plantar erythrodysesthesia (PPE) is also called hand-foot syndrome by reason of its location. The PPE is characterized by localized skin lesions on the palms and the soles. It may also spread to the skin of other sites like the groin or axillary region.
Treatment of cancer patients is still a huge challenge for oncologists. Usually cancer is diagnosed at an advanced stage. The treatment choice particularly important in patients with ovarian cancer, which is a chronic disease, and patients may be treated for many years. One of the treatment options is the use of PLD.
Current paper illustrates the skin complications observed in ovarian cancer patients during the treatment with PLD. The authors present the algorithm for prophylaxis and therapy of skin toxicity with particular emphasis on palmar-plantar erythrodysesthesia.
Material and methods
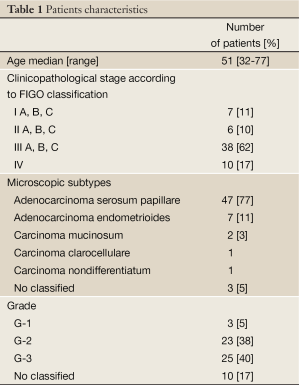
This retrospective analysis included medical records of ovarian cancer patients who were treated with LPD due to disease progression after prior therapy. The group comprised 61 woman treated in Clinical and Experimental Chemotherapy Department in Cancer Center and Institute in Gliwice, Poland between 2004-2009. Median age was 51 years (range, 32-77 years). All patients underwent cytoreduction surgery. All of them had microscopic confirmation of epithelial ovarian cancer. The most prevalent microscopic type was adenocarcinoma serosum papilare (77%) and adenocarcinoma serosum endometrioides (12%). In most patients IIIC and IV stage was diagnosed according to FIGO classification (61% and 16% respectively). Patients characteristics is presented in Table 1. All patients received paclitaksel and carboplatin based chemotherapy in post-surgery setting. The median time to relapse was 7 months. Relapse was defined according to RECIST criteria. The choice of consecutive chemotherapy type depended on patients general condition and response on previous chemotherapy. It depends also on the drugs availability in the country and the financing possibilities by the insurance. In 21 patients (34%) LPG was applied in second line chemotherapy, in 29 (48%) in third line, in the consecutive 10 (16%) patients in fourth line chemotherapy. LPG was given in dose 50 mg/m2 when it was used in monochemotherapy and in dose 40 mg/m2 when it was given in multidrug setting with carboplatin. Cycles were repeated every 28 days. Chemotherapy was continued until cancer progression or intolerable toxicity. Median number of cycles was 5 (range, 1-9). Clinical effectiveness was evaluated using RECIST criteria including Ca125 testing every 12 weeks. Toxicity was evaluated using NCI CTC criteria every cycle. Skin toxicity was evaluated using criteria presented in Table 2. Statistical analysis was performed using STATISTICA 8 PROGRAM. A selection of the particular variables for the risk of the occurrence of HFS was analyzed by Chi2 test with Yates’s correction. A P-value <0.05 was considered significant.
Full Table

Full Table
Results
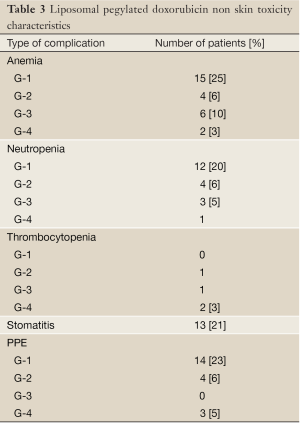
Median overall survival was 3.9 years (range, 1-12 years). Median time from ending of platinum derivatives to initiation PLD chemotherapy was 7 months. Any toxicity during PLD containing chemotherapy administration was observed in 80% of patients. The most common ones were hematological complications (neutropenia in 33%; anaemia in 44%; trombopenia in 7% of patients). We did not observe any cardiac complications. Stomatitis was observed in 21% of patients. Non skin toxicity is presented in Table 3. Skin toxicity occurred in 24 pts (40%). PPE was observed in 22 pts (36%). PPE occurred more often in patients who have previously received more than two lines of chemotherapy, P=0.09. PPE non significantly more frequently was observed in patients receiving PLD in monochemotherapy (75%) than in patients receiving PLD in multidrug setting (37%), P=0.18. Skin toxicity symptoms usually started after third chemotherapy cycles (9 patients -41%) and after second one (6 pts -10%). Three patients experienced complication just after first cycle in grade 1. We observed that the risk of PPE increased with the number of chemotherapy cycles including PLD, P<0.001. Grade 1 PPE intensity was observed in 23%, G-2 in 7%, G-3 in 5% of patients. In while in patients who develop anemia we observed PPE more frequently, P=0.038. Non skin toxicity led to chemotherapy dose reduction by 25% in 6 pts (10%). None but 2 pts (3%) hematological toxicity led to premature chemotherapy discontinuation. It was caused by neutropenia G-4 and trombopenia G-3 or G-4. PPE occurrence caused PLD dose reduction in 3 pts (5%). No patients required to premature chemotherapy completion due to PPE. In patients who develop stomatitis PPE was less frequently observed (52% vs. 87%), P=0.009. Similar situation was observed in the case of skin rash (29% vs. 95%), P=0.0001. In patients diagnosed with ovarian cancer recurrence in pelvic PPE occurred less frequently (48% vs. 77%), P=0.02. Authors are unable to explain this phenomenon.
Full Table
Patients’ age, menopausal status, performance status (ZUBRO-0 vs. ZUBROD-1-2) were not found to have significant influence on risk of PPE occurrence, P=0.48, P=0.23 and P=0.27, respectively.
EPP occurrence can not be considered neither as a prognostic or predictive factor for tumor response to PLD treatment. In patients who responded to treatment, PPE appeared in 44% of them compared to 31% of patients with tumor progression were, P=0.31.
Before starting chemotherapy containing PLD all patients were informed in detail about prophylaxis and possible home treatment. They were told the most important is to avoid tight clothing like socks and shoes as well as exposure to hot water and air. In the early stages of PPE the use of oily protective creams is effective. In more advanced lesions medical treatment was necessary. All of the patients received dexamethasone in chemotherapy premedication.
Discussion
Increasingly effective cancer treatment results in a significant prolongation of patients’ life. But now, the patients are no longer satisfy with life prolongation. They also demand good quality of life to enjoy the fullness of life in society and the family.
Ovarian cancer is a particular neoplasm. Despite the advanced stages at the time of diagnosis, in most tumors retains chemosensitivity. That allows for multiple chemotherapy which significantly prolongs survival. This forces an attention to minimizing the toxicity of treatment, which can lasts for many years. One of the therapeutic option is usage of derivate of antracycline - pegylated liposomal doxorubicin (PLD) which has improved the therapeutic index by reducing cardiac and hematologic toxicity. Nonetheless, it causes skin toxicity in form of PPE up to 50% (6). Our observations are generally consistent with reports of other authors. Any differences could result from a significant heterogeneity of the presented group of patients and retrospective nature of the analysis.
The pathogenic mechanism of PPE is still largely unknown. Some authors suggest it may be connected with PLD tendency to accumulate in keratinocytes (7). Others described that soles and palms are the areas exposed to risk of repeated mechanical trauma so it can results in achieve higher PLD concentration due to rich capillary network (8) or that may be associated with inflammatory process (9,10). That is why PPE occurs on skin areas vulnerable to warmth or pressure, not only palms and soles but also buttock, groin, under pendulous breast, axillae or inguinal regions (11). The clinical manifestation of PPE can be severe, the pts can develop such severe blisters, desquamation and ulcerations, that hospitalization becomes necessary. Distinctive increase in PPE often leads to treatment delay, doses reduction and in extreme cases of premature termination of treatment. This in turn reduces treatment effectiveness. PPE is typically observed after the second or third cycle of PLD (3).
Evaluation of patients, nursing and physician education is important and may aid in the promotion of prophylactic intervention, early recognition and best practices in skin toxicity management, and improving patient’s quality of life. So the key is early patients’ and their family members’ information about the possible skin complications, the prevention and simple treatment at home.
Prevention strategy include avoidance exposure to heat, hot water or air (showers, sun exposure). Patients should not, if possible, wear gloves or socks and stay in cool areas. During the hottest summer to take cold baths, use moisturizing creams and lotions (12,13). It is also important to avoid daily activities or exercises that may result in injury to the skin feet (running) or hands (physical work).
Dexamethasone should be given in PLD premedication in the dose 8mg twice daily through 5 days, which delays and reduced symptoms of PPE.
Some authors argue that pyridoxine may provide relief of PPE (14). But rationale for its use is doubtful, there are no III phase trials on this field. The dosage suggestions are 50-150 mg per day.
In the topical treatment effective in relieve PPE may be usage of lotions with aloe vera or lanoline or green tea or dimethyl sulfoxide (DMSO) (15). Topical administration of 99% DMSO few times a day may accelerate healing of PPE symptoms.
Others drugs, like COX-2 inhibitors, cod liver oil are still under investigations.
In more severe grade toxicity chemotherapy doses modification is necessary.
Although the findings are limited due to the retrospective observational nature, but you can answer important questions about the treatment of skin complications.
Conclusions
Today’s chemotherapy gives to many incurable cancer patients opportunity for significantly prolongation of their life. However, it gives different and sometimes severe complications, which may substantially reduce quality of life. Skin toxicity is not life threatening, nonetheless may significantly limit the possibility to continue the treatment. Good communication with patients and their families, close cooperation with family doctors, palliative care and dermatologists is essential for prompt and effective treatment of skin toxicity.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- D’epiro S, Salvi M, Mattozzi C, et al. Gemcitabine-induced extensive skin necrosis. Case Rep Med 2012;2012:831616.
- Campanati A, Berardi R, Onofri A, et al. A novel approach to manage skin toxicity caused by therapeutic agents targeting epidermal growth factor receptor. Ann Oncol 2012;2:1081-2. [PubMed]
- Martschick A, Sehouli J, Patzelt A, et al. The pathogenetic mechanism of anthracycline-induced palmar-plantar erythrodysesthesia. Anticancer Res 2009;2:2307-13. [PubMed]
- Goram AL, Richmond PL. Pegylated liposomal doxorubicin: tolerability and toxicity. Pharmacotherapy 2001;2:751-63. [PubMed]
- Eng C, Mauer AM, Fleming GF, et al. Phase I study of pegylated liposomal doxorubicin, paclitaxel, and cisplatin in patients with advanced solid tumors. Ann Oncol 2001;2:1743-7. [PubMed]
- Charrois GJ, Allen TM. Multiple injections of pegylated liposomal Doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther 2003;3:1058-67. [PubMed]
- Fitzpatrick JE. The cutaneous histopathology of chemotherapeutic reactions. J Cutan Pathol 1993;2:1-14. [PubMed]
- Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol 2000;3:1475-80. [PubMed]
- DeSpain JD. Dermatologic toxicity of chemotherapy. Semin Oncol 1992;2:501-7. [PubMed]
- Skelton H, Linstrum J, Smith K.. Host-vs.-altered-host eruptions in patients on liposomal doxorubicin. J Cutan Pathol 2002;2:148-53. [PubMed]
- Lorusso D, Di Stefano A, Carone V, et al. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann Oncol 2007;2:1159-64. [PubMed]
- Molpus KL, Anderson LB, Craig CL, et al. The effect of regional cooling on toxicity associated with intravenous infusion of pegylated liposomal doxorubicin in recurrent ovarian carcinoma. Gynecol Oncol 2004;2:513-6. [PubMed]
- Edwards SJ. Prevention and treatment of adverse effects related to chemotherapy for recurrent ovarian cancer. Semin Oncol Nurs 2003;2:19-39. [PubMed]
- Vail DM, Chun R, Thamm DH, et al. Efficacy of pyridoxine to ameliorate the cutaneous toxicity associated with doxorubicin containing pegylated (Stealth) liposomes: a randomized, double-blind clinical trial using a canine model. Clin Cancer Res 1998;1:1567-71. [PubMed]
- Lopez AM, Wallace L, Dorr RT, et al. Topical DMSO treatment for pegylated liposomal doxorubicin-induced palmar-plantar erythrodysesthesia. Cancer Chemother Pharmacol 1999;2:303-6. [PubMed]


