Stereotactic body radiation therapy for metastases to the kidney in patients with non-small cell lung cancer: a new treatment paradigm for durable palliation
Introduction
The great successes of stereotactic radiosurgery (SRS) for intracranial metastases prompted logical assessment as to whether similar treatments could be delivered to other body sites. The advent of stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), consists of several hallmark characteristics shared with SRS. First, SBRT mandates substantial attention to patient immobilization and accountability for tumor motion between, and in many cases, during, treatments. Owing to the utilization of many precise radiation beams, SBRT affords superior conformity to the target volume, resulting in a steep dose drop-off of irradiation dose between the tumor volume and surrounding organs at risk. Lastly, SBRT most classically is accomplished in five or fewer treatments of large ablative doses delivered during each radiation fraction, allowing for optimal patient convenience.
These properties of SBRT have allowed its capabilities to be translated into treatment of a variety of tumors in many different areas of the body. The most well characterized treatment with SBRT is that of primary lung cancer, for which SBRT has been shown to be a safe and effective treatment for medically inoperable early stage lung cancer (1-4), operable early stage lung cancer (5-7), large primary lung tumors greater than 5 cm (8-10), and early stage small cell lung cancer (11-13). SBRT is also increasingly being used in stage IV disease for palliative purposes (14-16) and even for definitive therapy in oligometastatic disease (17-19). SBRT for oligometastatic disease is generally associated with limited toxicities and high rates of local control, which in selected patients can allow for delays until the need for systemic therapy, an improvement in progression free survival, or potentially even an improvement in overall survival (20,21). However, studies remain hampered by a lack of uniformity in patient selection and aggressiveness of treatment (both regarding number and type of metastases as well as patient-related factors such as age and performance status) (22,23).
Although metastasis to the kidney is rare, with only a few case reports described thus far (24-28), current management is largely limited to extrapolation from SBRT in primary renal cell carcinoma (RCC) cases (29,30). Hence, there is a clear need for further clinical experiences of utilizing SBRT in these circumstances and assessing outcomes and toxicities. The high conformality of SBRT makes it an attractive option to treat renal lesions, including in patients with some degree of renal dysfunction (31) or sparing parts of the same kidney as the lesion, which is particularly useful in patients with one functioning kidney or patients in whom kidney function has been reduced from the metastasis itself or from the effects of cytotoxic chemotherapy (32).
In the absence of more experiences, and multiple aforementioned cases documenting kidney metastases without examining clinical management (24,26), this is the largest series to date of patients with renal metastases, as well as the only report examining SBRT treatment for such conditions, including associated treatment course, outcomes, and toxicities.
Methods
This is an Institutional Review Board (University of Pennsylvania)-approved retrospective analysis of patients with biopsy-proven, metastatic cancer involving the kidney that received SBRT. Ethics Committee approval was not required per institutional guidelines. Primary RCCs were not included in this analysis. Chart review was performed in order to determine clinical course, including outcomes and toxicities, in these patients.
Prior to radiotherapy treatment, patients underwent four-dimensional CT simulation using body fixation and immobilization devices, with supine positioning and arms abducted. Intravenous contrast was given unless renal function precluded administration. Pre-simulation images, including PET-CT scans and MRIs, were fused to simulation images to aid in target delineation. On the free-breathing scan, the gross tumor volume was contoured; an internal target volume was delineated based on expansion of the gross tumor volume based on tumor motion from eight different breathing phases, followed by an additional 0.3–0.5 cm margin expansion to form the planning target volume (PTV). Normal tissues were contoured in accordance with Radiation Therapy Oncology Group (RTOG) guidelines (33). Dose constraints used were from many sources, including QUANTEC (34), SBRT dose tolerance publications (35), the RTOG 0631 protocol (36), and other previous reports discussed on SBRT for primary RCC. The prescribed dose was required to cover at least 95% of the PTV.
Immediately prior to each treatment, patients were instructed not to take anything by mouth so as to avoid gross bowel distension. Daily image guidance with cone-beam CTs pre-treatment was performed before each fraction.
During radiotherapy, acute toxicities and tolerance of treatment were assessed. Acute and late toxicities were evaluated at each follow-up, with visits occurring 1 month following SBRT completion, 3 months following SBRT completion, and generally every 2–4 months thereafter depending on the clinical circumstance of each patient, including subsequent therapies. Imaging, generally with computed tomography (CT) with intravenous contrast but otherwise positron emission tomography-CT (PET-CT), was performed prior to each follow-up visit with the exception of the first follow-up visit that occurred 1 month following SBRT completion. Primary outcome measures included pain and symptomatic response, as well as disease controls and treatment toxicities as assessed by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Results
Case 1
A 57-year-old female experienced a mechanical fall with persistent chest pain thereafter. This led to imaging, which illustrated a 4×4 cm right suprahilar mass obliterating the right upper lobe apical segmental bronchus. There was FDG avidity in the ipsilateral mediastinum and bilateral hila. Pathology showed adenocarcinoma of lung primary. Concurrent chemoradiation was then started, with radiotherapy to a total dose of 66.6 Gy; she received 3 cycles of maintenance carboplatin/paclitaxel following thoracic radiotherapy completion. Approximately 20 months thereafter, she developed headaches and confusion. Brain MRI confirmed the presence of multiple brain metastases; there was no systemic disease noted on imaging. She underwent resection of the largest (4 cm) right frontal lobe lesion, followed by whole brain radiotherapy (3,500 cGy in 14 fractions). She then received chemotherapy (carboplatin, pemetrexed, and bevacizumab), which was discontinued after 3 months owing to a poorly-healing scalp wound.
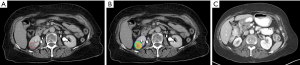
The patient had stable disease for the next 7 months, when imaging revealed a new 1.6×1.5 cm enhancing mass along the posterior aspect of the lower pole of the right kidney (Figure 1A). She noted new onset intermittent right flank pain beginning approximately 1 month prior to that imaging. She underwent a course of SBRT to the isolated renal lesion to a dose of 24 Gy in 3 fractions (Figure 1B). She did not report side effects from this therapy. Four months later, by which time the wound had healed, pemetrexed and bevacizumab was restarted for a total of 5 months. Six months thereafter, PET-CT revealed complete metabolic response of her renal metastasis and excellent anatomical response (Figure 1C). At that time, she noted complete pain response. She subsequently developed a right hepatic lobe metastasis and is being treated with single-agent pemetrexed.
Case 2
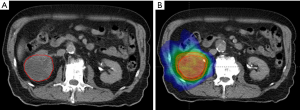
A 77-year-old man was to undergo stent placement for an abdominal aortic aneurysm (AAA). During preoperative workup, imaging revealed a 2.7 cm pleural-based left lower lobe mass that was FDG avid without other foci of disease. Following AAA repair, restaging scans demonstrated slight increase in size to 3.1 cm, without pathologic lymphadenopathy or distant disease. He declined definitive surgery and underwent SBRT to the lung lesion to 50 Gy delivered in 5 fractions. The patient remained without evidence of disease for one and a half years, when he presented with early satiety and diffuse abdominal pain (right greater than left) requiring narcotics. Imaging revealed a malignant-appearing right renal lesion (Figure 2A). CT-guided biopsy revealed this to be a squamous cell carcinoma morphologically consistent with a lung metastasis, instead of a primary RCC. He again wished to avoid surgery, and SBRT was delivered to his only site of known disease to 30 Gy in 5 fractions (Figure 2B). He reported grade 1 fatigue that persisted for several months, as well as grade 1 nausea not requiring intervention that was intermittent and lasted only a day beyond treatment. He achieved an early partial pain response that was sustained throughout the course of his follow-up and allowed for him to come off of narcotics.
He was without evidence of disease for the following 8 months, until imaging revealed an ipsilateral perinephric metastatic implant. He then commenced pembrolizumab for this progression.
Case 3
A 69-year-old male initially presented with a nonproductive cough. Further imaging demonstrated an 8×8 cm left lower lobe mass with two enlarged ipsilateral mediastinal nodes and an ipsilateral hilar node. Pathology revealed poorly-differentiated bronchogenic carcinoma with squamous features. MRI brain revealed five small cerebellar metastases. Multidisciplinary recommendation was to proceed with definitive oligometastatic therapy. Concurrent chemoradiation was commenced; however, during treatment he began experiencing right hip pain, and imaging revealed a right supra-acetabular lytic lesion with associated pathologic fracture. He received palliative radiotherapy to the supra-acetabular lesion along with the completion of his thoracic radiotherapy. He was also treated with SRS to the intracranial metastases.
Three months later, he underwent restaging PET-CT and brain MRI, which showed a new right occipital lobe metastasis along with fluorodeoxyglucose (FDG)-avid disease in the cecum, small bowel, a left cervical lymph node, and the lower pole of the right kidney.
He then continued on chemotherapy and received SRS to the right occipital metastasis. A month later, he was put on nivolumab, which was continued for the next 3 months. However, imaging demonstrated increasing size of the existing abdominal disease, and new mesenteric lymph nodes and a new right hepatic lesion. When nivolumab was stopped, he began to have diffuse abdominal pain that started from waxing/waning lower abdominal pain. At that point, imaging showed progression of mesenteric and small bowel metastasis now causing a bowel obstruction, along with a significant increase in a right renal lesion, which began involving the collecting system without noted hydronephrosis.
He received palliative radiotherapy to the bowel lesion, after which the bowel obstruction clinically improved, but he continued to localize pain in the right flank. He was then put on a clinical trial of pembrolizumab. After multidisciplinary discussion based on the risks of local progression and good response to radiotherapy in the abdomen, it was elected to treat the renal lesion with SBRT, and to monitor the mesenteric lymphadenopathy and liver metastasis.
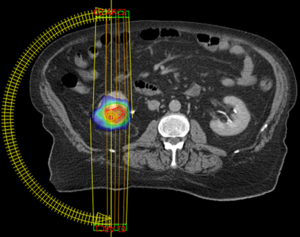
He then underwent a course of SBRT to the right kidney lesion (Figure 3), administered in three fractions of 800 cGy each. He tolerated this well without acute toxicities caused by SBRT and at one month following SBRT denied any persistence of abdominal pain. Three months after SBRT completion, he passed away from worsening intracerebral disease.
Case 4
A 72-year-old female presented with cough and was found to have a dominant right upper lobe lung cancer and multiple bilateral lung nodules consistent with thoracic-only stage IV disease. Pathology revealed adenocarcinoma of lung origin. She was initially treated with systemic therapy. Nine months after treatment initiation, while on maintenance chemotherapy, she developed progression in the right lower lobe, along with a new, asymptomatic right renal lesion. Biopsy of the renal lesion confirmed lung metastasis. Palliative radiotherapy was administered to the right lower lobe area, along with the renal mass (24 Gy in 3 fractions). She did not report side effects from SBRT. She was observed off of systemic therapy following radiation therapy, and imaging 3 months following SBRT revealed significant partial response by size to her kidney metastasis.
Discussion
This report is the largest known series of patients with renal metastases, as well as the only known series of these patients treated with SBRT. Based on this limited experience, we found SBRT to be a safe and effective treatment modality, making it an important tool in the palliative care setting.
Although there are other radiotherapy techniques available to utilize in the palliative setting, SBRT was elected in these patients for several reasons. First, its ability to deliver ablative doses in 5 or fewer fractions is both radiobiologically and logistically advantageous as compared to conventionally-fractionated (or even hypofractionated) photon treatment. These advantages can allow for improvements in local control and in patient convenience, respectively. SBRT is also highly conformal, which was particularly important in these patients, either due to their large tumor sizes or being able to better spare bowel and other nearby organs-at-risk from irradiation. This property is similar to proton and particle therapy, which have previously been shown compared with photons to better protect nearby organs-at-risk in patients with non-small cell lung cancer (NSCLC) (37,38), the diagnosis of our study population, as well as those with gastrointestinal malignancies (39), the region our study population was treated to. However, SBRT is generally more cost-effective in the palliative setting, and SBRT is also offered at most modern radiotherapy centers, unlike particle therapy. There are also currently no published experiences with particle therapy in this setting, and given renal tumors have intrafractional motion, there is a potential concern of an interplay effect and thus risk of undertreating the tumor volume that has been reported for SBRT (40) but is an even bigger risk with particle therapy (41). However, SBRT does have disadvantages as compared to conventionally-fractionated therapy (e.g., possibly less cost-effective and/or potential toxicities when delivering higher doses per fraction to nearby organs-at-risk) and particle therapy (e.g., less conformal).
There are several reflections from our analysis. First, SBRT did not cause appreciable toxicities in a patient population with advanced disease for whom a primary oncologic principle is to “do no harm”. This is essential to confirm the value of palliative SBRT in any setting. Second, in all cases, SBRT provided satisfactory local control, without in-field recurrences noted during these relatively early time points. Indeed, as seen herein, when SBRT is applied to the kidney, it is often utilized as a means to control local disease progression. In this manner, we propose the term “definitive palliation” to represent the expectation of “definitive” local control (that is, a complete response or lack of future progression within the irradiated area) for the remainder of life of a patient. Third, patients presenting with renal neoplasms can often present with vague symptoms such as early satiety, dull abdominal or back pain, and/or gastrointestinal symptoms. In our series, 3 patients presented with pain and 1 with early satiety. In all cases, the symptoms improved, with 2 of the 3 patients having a complete pain response, and the remaining patient a partial response that allowed for the discontinuation of narcotics. This further lends support for the value of SBRT for symptomatic palliation purposes. Fourth, we recognize that tumor response and symptomatic relief in this series might have been confounded by receipt of chemotherapy. However, several patients had progressed on chemotherapy, which is why SBRT was recommended in the first place.
More broadly, selecting patients for relatively aggressive treatment, including renal SBRT, is a prime concern going forward. However, SBRT might be particularly useful for kidney lesions when radiation therapy is needed, since even palliative low doses of irradiation can result in nausea and other morbidities due to the close proximity of renal lesions to other normal critical structures like the liver, stomach, and bowel. In previous studies, patients have been analyzed according to known poor prognostic factors affecting survival after SBRT for oligometastases, such as more than one metastasis and bone metastases (42). Additionally, four variables studied in a Belgian report (43)—non-adenocarcinoma histology, male gender, synchronous oligometastatic disease, intracranial metastases—revealed that median survival with 0, 1, 2, 3, and 4 risk factors were 40, 29, 23, 9, and 4 months respectively. Herein, most patients had 3–4 risk factors, which is reflected in their generally lower survival.
Our results are similar to previous work. Although every case report of kidney metastases have been from NSCLC, only three reports have demonstrated specific clinical course and treatment. Whereas Derweesh et al. (25) noted new gross hematuria in a patient 2 years after localized NSCLC treated with surgery alone, Cai et al. (28) described a patient with a 5 cm mass (squamous cell carcinoma) with pathologic N2 involvement treated with adjuvant chemoradiation (cisplatin/vinblastine and 50 Gy) with a 10-month disease-free interval before microscopic hematuria commenced further workup. This patient reportedly refused nephrectomy and was treated with radiosurgery, but there are no details after treatment mentioned. Lastly, Barry-Brooks et al. (27) described a patient with a left upper lobe mass invading the mediastinum at presentation; although no mediastinal lymphadenopathy was evident on PET scan, the metastatic renal lesion (adenocarcinoma) was apparent at initial presentation; chemoradiotherapy in this patient resulted in survival of 1 year. Taken together, metastases to the kidney may be most common from lung cancer, without clear histological or stage-based predilection, although reports of cases are too sparse to make definitive conclusions.
It is useful to examine renal function after SBRT to the kidney. Jackson and colleagues (44) used SPECT-CT to determine that renal function decreased 34% at 3 months and 43% at 12 months, with minimal decrease thereafter. These results are useful to apply to similar patients in order to coordinate multidisciplinary management with nephrologists managing renal parameters, although differences in tumor extent, treatment technique, and their use of single-fraction treatment need to be accounted for when generalizing these functional results to our current study population. Further data regarding the impact of different fractionation regimens on these parameters is warranted for assessing both preservation of normal kidney function and achieving optimal local control. Moreover, a study of seven RCC patients (32), in which the RCC occurred in the only remaining (functional) kidney, reported that serum creatinine in five patients remained at pre-SBRT levels. Dose-volume analysis of the data yielded mean ipsilateral renal doses of 28–42% of the prescribed dose, with an exception of 11% in a very small lesion. V15 (volume of kidney receiving at least 15 Gy) was from 20–37% (small lesion exception of 5%). Larger lesions tended to show larger parameters of both renal mean and V15 doses.
Additional information is needed on the potential benefits and risks of SBRT in renal neoplasms, particularly for metastatic renal lesions. For instance, owing to the dearth of clinical scenarios of even primary RCC, SBRT experiences are also limited and hence technical data are also lacking. Although the kidneys are anchored to the retroperitoneum by Gerota’s and retroperitoneal fascia, there is evidence that there is some degree of respiratory (diaphragmatic motion) influence. One report showed < 10 mm movements in the anteroposterior and lateral directions with free breathing, yet up to four times as much movement with forced shallow and deep breathing (45), the latter of which can result in up to 50% loss in tumor coverage if not accounted for during planning (46). However, there is also evidence that left kidney movement is more limited than right kidney movement (47). Although these data are compelling, further reports are encouraged to validate and verify these data, which predictably can have impacts on SBRT treatment planning for lesions of the kidney.
In summary, we demonstrate that SBRT for metastatic cancers to the kidney is well-tolerated and safe, and it can provide good symptomatic relief and early local control. These data lend support to its use in these patients, not only in palliative settings, but also potentially for oligometastatic settings in an attempt to improve clinical outcomes as well.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is an Institutional Review Board (University of Pennsylvania)-approved retrospective analysis of management of patients with biopsy-proven, metastatic cancer involving the kidney that received SBRT. Ethics Committee approval was not required per institutional guidelines.
References
- Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007;25:947-52. [Crossref] [PubMed]
- Simone CB 2nd, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784-90. [Crossref] [PubMed]
- Verma V. Lung cancer: Implementing lung-cancer screening--oncological “grey areas”. Nat Rev Clin Oncol 2015;12:256-7. [Crossref] [PubMed]
- Verma V, Zhen W. Treatment Costs of Early-Stage Lung Cancers Detected by Low-Dose Computed Tomography Screening. Int J Radiat Oncol Biol Phys 2015;93:207-8. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Verma V. Stereotactic Radiotherapy Versus Surgery for Early-Stage Operable Lung Cancer: More Questions Than Answers. J Natl Compr Canc Netw 2015;13:1293-5. [Crossref] [PubMed]
- Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:688-96. [Crossref] [PubMed]
- Verma V, McMillan MT, Grover S, et al. Stereotactic Body Radiation Therapy and the Influence of Chemotherapy on Overall Survival for Large (≥5 Centimeter) Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- Verma V, Shostrom VK, Zhen W, et al. Influence of Fractionation Scheme and Tumor Location on Toxicities After Stereotactic Body Radiation Therapy for Large (≥5 cm) Non-Small Cell Lung Cancer: A Multi-institutional Analysis. Int J Radiat Oncol Biol Phys 2017;97:778-85. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Zhen W. Stereotactic Radiotherapy for Stage I Small Cell Lung Cancer. Oncologist 2016;21:131-3. [Crossref] [PubMed]
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer 2017;103:11-6. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Allen PK, et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:362-371. [Crossref] [PubMed]
- Bedard G, McDonald R, Poon I, et al. Stereotactic body radiation therapy for non-spine bone metastases--a review of the literature. Ann Palliat Med 2016;5:58-66. [PubMed]
- Knisely J, Sahgal A, Lo S, et al. Stereotactic radiosurgery/stereotactic body radiation therapy-reflection on the last decade's achievements and future directions. Ann Palliat Med 2016;5:139-44. [Crossref] [PubMed]
- Jones JA, Simone CB 2nd. Palliative radiotherapy for advanced malignancies in a changing oncologic landscape: guiding principles and practice implementation. Ann Palliat Med 2014;3:192-202. [PubMed]
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 2010;5:1091-9. [Crossref] [PubMed]
- Baumann BC, Nagda SN, Kolker JD, et al. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol 2016;114:65-9. [Crossref] [PubMed]
- Patel AN, Simone CB 2nd, Jabbour SK. Risk factors and management of oligometastatic non-small cell lung cancer. Ther Adv Respir Dis 2016;10:338-48. [Crossref] [PubMed]
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824-30. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100-7. [Crossref] [PubMed]
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [Crossref] [PubMed]
- Yoshino I, Yohena T, Kitajima M, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg 2001;7:204-9. [PubMed]
- Derweesh IH, Ismail HR, Magi-Galluzzi C, et al. Non-small cell lung carcinoma metastatic to the kidney. Can J Urol 2006;13:3281-2. [PubMed]
- Finke NM, Aubry MC, Tazelaar HD, et al. Autopsy results after surgery for non-small cell lung cancer. Mayo Clin Proc 2004;79:1409-14. [Crossref] [PubMed]
- Barry-Brooks M, Yoo DC, Chaump M, et al. Non-small cell lung cancer with unsuspected distant metastasis to the kidney seen on PET/CT. Med Health R I 2012;95:144-6. [PubMed]
- Cai J, Liang G, Cai Z, et al. Isolated renal metastasis from squamous cell lung cancer. Multidiscip Respir Med 2013;8:2. [Crossref] [PubMed]
- Siva S, Pham D, Gill S, et al. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int 2012;110:E737-43. [Crossref] [PubMed]
- Pham D, Thompson A, Kron T, et al. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys 2014;90:1061-8. [Crossref] [PubMed]
- Lo CH, Huang WY, Chao HL, et al. Novel application of stereotactic ablative radiotherapy using CyberKnife(®) for early-stage renal cell carcinoma in patients with pre-existing chronic kidney disease: Initial clinical experiences. Oncol Lett 2014;8:355-60. [PubMed]
- Svedman C, Karlsson K, Rutkowska E, et al. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol 2008;47:1578-83. [Crossref] [PubMed]
- Upper abdominal normal organ contouring consensus guidelines. Available online: https://www.rtog.org/CoreLab/ContouringAtlases/UpperAbdominalNormalOrganContouringConsensusGuidelines.aspx
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [Crossref] [PubMed]
- Grimm J, LaCouture T, Croce R, et al. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 2011;12:3368. [Crossref] [PubMed]
- RTOG 0631 protocol information. Available online: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0631
- Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol 2016;11:66. [Crossref] [PubMed]
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014;20:427-32. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB 2nd, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Zou W, Yin L, Shen J, et al. Dynamic simulation of motion effects in IMAT lung SBRT. Radiat Oncol 2014;9:225. [Crossref] [PubMed]
- Lin L, Souris K, Kang M, et al. Evaluation of motion mitigation using abdominal compression in the clinical implementation of pencil beam scanning proton therapy of liver tumors. Med Phys 2017;44:703-12. [Crossref] [PubMed]
- Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012;83:878-86. [Crossref] [PubMed]
- de Vin T, Engels B, Gevaert T, et al. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol 2014;25:467-71. [Crossref] [PubMed]
- Jackson P, Foroudi F, Pham D, et al. Short communication: timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial (99m)Tc-DMSA SPECT/CT. Radiat Oncol 2014;9:253. [Crossref] [PubMed]
- Pham D, Kron T, Foroudi F, et al. A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technol Cancer Res Treat 2014;13:315-23. [PubMed]
- Pham D, Kron T, Foroudi F, et al. Effect of different breathing patterns in the same patient on stereotactic ablative body radiotherapy dosimetry for primary renal cell carcinoma: a case study. Med Dosim 2013;38:304-8. [Crossref] [PubMed]
- Siva S, Pham D, Gill S, et al. An analysis of respiratory induced kidney motion on four-dimensional computed tomography and its implications for stereotactic kidney radiotherapy. Radiat Oncol 2013;8:248. [Crossref] [PubMed]


