Guidelines on cancer pain management (2011 Edition)
1. Introduction
Pain is one of the most common symptoms of cancer patients and causes serious disruption in patients’ quality of life. The incidence of cancer pain was approximately 25% among the newly diagnosed cancer patients but can reach 60-80% in the those with advanced diseases, of whom one third were with severe pain. Cancer pain, if not alleviated, can lead to extreme discomfort and cause or aggravate symptoms including anxiety, depression, fatigue, insomnia, and loss of appetite, posing serious impacts on patients’ daily activities, self-care, social interactions, and overall quality of life.
The Guidelines on Cancer Pain Management was formulated with an attempt to further standardize the cancer pain management, optimize the diagnosis and treatment of major diseases, improve the practices of cancer pain management in medical institutions, improve the quality of life of cancer patients, and guarantee the quality and safety of health care in China.
2. Causes, mechanism, and types of cancer pain
2.1 Causes of cancer pain
The cancer pain can by caused by various causes:
- Caused by cancer itself: the cancer may invade surrounding tissue, or spread (metastasize) to distant sites (e.g., bone) in the body;
- Caused by anti-cancer treatment: it may commonly occur after surgery, invasive examinations, radiation therapy, and cytotoxic chemotherapy;
- Caused by non-tumor-related factors: it may be caused by co-morbidities, complications, and other factors.
2.2 Mechanism and classification of cancer pain
- (I) The pain can be classified into two types according to pathophysiological mechanisms: nociceptive and neuropathic.
- (i) The nociceptive pain is caused by the injured visceral or somatic tissues due to noxious stimuli. The nociceptive pain is related with the actual tissue injury or potential injury, representing the body’s physiological signal conduction and response of the pain nerve towards the injuries. The nociceptive pain includes visceral or somatic pain. The somatic pain can be blunt, sharp or compressive. The visceral pain, on the contrary, is often presented as diffuse pain or cramping pain that can not be accurately located.
- (ii) The neuropathic pain is caused by the the abnormal nerve impulses produced in the pain afferent fibers and/or pain center due to the injury of peripheral or central nerves. The neuropathic pain is often featured by stabbing, burning, electric, shooting, numbness, tingling, and radiation pain. Phantom limb, central bearing-down pain, and bursting pain are often accompanied with spontaneous pain, allodynia, hyperalgesia, and pain hypersensitivity. Post-treatment pain also belongs to neuropathic pain.
- (II) The pain is divided into acute pain and chronic pain according to an arbitrary interval of time since onset. Most cancer pain is chronic. Compared with the acute pain, the chronic pain usually lasts a long period of time, with unidentified causes. The intensity of pain may not be directly associated with tissue injury and can be accompanied by hyperalgesia, allodynia, and poor response to conventional pain therapy. The mechanisms of chronic pain and acute pain share some common features but naturally have many differences. During the development of chronic pain, the basic signaling modulation process for the nociceptive pain is involved; meanwhile, other mechanisms of neuropathic pain including the hyperactive nociceptors, ectopic electrical activity of the injured nerve, hypersensitivity of the central mechanisms for pain transmission, abnormal expressions of ion channels and receptors, and the remodeling of the central nervous system.
3. Evaluation of cancer pain
Evaluation of cancer pain is the premise of appropriate and effective pain treatment. Cancer pain evaluation should be conducted in a routine, quantified, comprehensive, and dynamic manner.
3.1 Routine
The routine cancer pain evaluation means that the medical staff actively ask the cancer patient about the possible presence of pain, routinely assess the pain conditions, and record the results in the medical records. It should be completed within 8 hours after admission. For patients with symptoms of cancer pain, pain evaluation should be included in the routine nursing practices (e.g., monitoring and documentation). The routine pain evaluation should also differentiate the causes of any breakthrough pain such as those seen in urgent cases including pathologic fractures, brain metastases, infections, and intestinal obstruction.
3.2 Quantified
The quantified evaluation of cancer pain refers the use of pain assessment scales for determining the patients’ subjective feelings about the pain. It requires close cooperation between patients and healthcare providers. During the quantified evaluation of pain, the most severe and mildest pain a patient feels in the past 24 hours are most important; meanwhile, the intensity of pain under the general conditions should also be assessed. The quantified evaluation of pain should be completed within 8 hours after admission. Three commonly used methods include Numerical Rating Scale (NRS), face pain scales, and verbal rating scales (VRS).
- (I) The Numeric Rating Scale (NRS) is an 11-point scale for patient self-reporting of pain (Figure 1). The pain level is scored from 0 to 10, with “0” as “no pain” and “10” as “most severe pain”. The patient is asked to select a number that most appropriately represent his/her pain severity; alternatively, a doctor or nurse asks the patient “How severe is your pain” and then select a corresponding number. The numbers used to rate the pain level are as follows: 0, no pain; 1-3, mild pain; 4-6, moderate pain; and 7-10, severe pain.
- (II) Faces Pain Scale: Using the face pain rating scales (Figure 2), the medical staff assesses the pain via facial expression. It is feasible for patients with difficulties in communication (e.g., children and the elderly), with culture/language barriers, and/or with other communication obstacles.
- (III) Verbal rating scales (VRS): the pain is classified as mild, moderate, and severe based on the patients’ complaints of their pain.
- (i) Mild: pain exists, but tolerable; the patient has normal living activities, without disrupted sleep;
- (ii) Moderate: the pain becomes obvious and intolerable; the patient seeks analgesic drugs, with disrupted sleep;
- (iii) Severe: the pain is severe and intolerable. The patient usually seeks analgesic drugs, with seriously disrupted sleep. Autonomic disorders or passive position may also exist.
3.3 Comprehensive
The comprehensive evaluation of cancer pain requires a thorough assessment of the pain and its relevant diseases, including the causes and types of pain (somatic, visceral or neuropathic), onset of pain (nature of the pain as well as factors that may aggravate or mitigate it), pain treatment, functions of major organs, psychological/mental disorders, family and social supports, and previous histories (e.g., metal disease, drug abuse, etc.). The first comprehensive assessment should be carried out within 24 hours after admission. During the treatment, a second comprehensive assessment should be arranged 3 days after analgesic treatment or after a stable remission is achieved; in principle, such comprehensive assessment should be less than 2 times per month.
The comprehensive assessment of cancer pain is usually based on the Brief Pain Inventory (BPI) (see Annex), assessing the pain and its impacts on the patient’s mood, sleep, activity, appetite, daily life, ability to walk, and other factors that is related with the quality of life. Efforts should be made to encourage the patients to express their needs and concerns about analgesic treatment; furthermore, it is important to formulate the targets to optimize the patients’ functions and quality of life by providing tailored pain treatment.
3.4 Dynamic
The dynamic assessment of cancer pain refers to the continuous and dynamic monitoring of the changes in the pain-related symptoms in cancer patients, including the intensity of pain, change of the pain nature, onset of breakthrough pain, factors that may relieve or aggravate pain, as well as the adverse reactions of analgesic treatment. Dynamic assessment is particularly important for dose titration during analgesic treatment. During the analgesic treatment, the medication types and dose titration, the intensity of pain, and changes in disease condition should be recorded.
4. Management of cancer pain
4.1 Principles of management
Based on the principles of multidisciplinary treatment, tailored analgesic treatment should be provided to continuously and effectively eliminate the pain, prevent/control adverse drug reactions, and reduce the psychological burden associated with pain and anti-pain treatment, so as to maximize the quality of life in cancer patients.
4.2 Therapies
The therapies for cancer pain include etiological treatment, pharmaceutical therapies, and non-pharmaceutical therapies.
4.2.1 Etiological treatment
The treatment is targeted at the causes of cancer pain. The main causes of cancer pain include cancer itself, complication, and others. Anti-cancer treatment including surgery, radiotherapy, and chemotherapy, can be provided for cancer patients to relieve cancer pain.
4.2.2 Pharmaceutical therapies
- (I) Principles. The use of analgesics can be arranged according to the five basic principles in the guidelines of the World Health Organization (WHO):
- (i) “By mouth”. If possible, analgesics should be given by mouth. For patients who are not feasible oral administration, patient-controlled analgesia (e.g., transdermal patches) and subcutaneous morphine may also be applied.
- (ii) “By the bladder”. According to the pain density, the analgestics should be used sequentially.
- a) Mild pain: Non-steroidal anti-inflammatory drugs (NSAID) may be applied;
- b) Moderate pain: an opioid for mild or moderate pain may be used, or in combination with NSAID;
- c) Severe pain: an opioid for severe pain may be used, or in combination with NSAID.
The combination of NSAID with an opioid can enhance the analgesic effect of the opioid and lower its dosage. An opioid for severe pain can also be applied for patients with mild or moderate pain only if it can reach a good analgesic effect with no serious adverse reactions. Antidepressants and anticonvulsants are the drugs of choice for neuropathic pain.
- (iii) “By the clock”. The analgesics should be administered at pre-determined intervals. Dosing by the clock is helpful to maintain stable and effective plasma drug concentration. Along with the wide application of controlled/sustained release formulations, the controlled/sustained-release opioids have been used as the basic medications. Immediate-release (IR) opioids can be used for symptomatic treatment during titration and breakthrough pain.
- (iv) “For the individual”. Individualized regimen should be formulated based on the disease condition and the dose of anti-pain drugs. There are no standard doses for opioid drugs. The “right” dose is the dose that relieves the patient’s pain. Meanwhile, the need for drug combination should also be considered for patients with neuropathic pain.
- (v) “Attention to detail”. Patients on analgesic treatment should be carefully monitored. The pain relief and body response should be closely observed. Any drug interaction should be timely detected. Effective measures should be taken to minimize the adverse drug reactions to improve the patient’s quality of life.
- (II) Selection and use of drugs. In accordance with the intensity and nature of the pain, ongoing therapies, and co-morbidities of a caner patient, analgesics and adjuvant drugs should be appropriately selected. Meanwhile, the dose and frequency of the medications should be tailored to optimize the analgesic effectiveness and prevent/reduce adverse reactions.
- (i) Non-steroidal anti-inflammatory drugs (NSAIDs) NSAIDs belong to the basic drugs for cancer pain. Different NSAIDs have a similar mechanism for relieving pain and inflammation and are often used for mild pain, or, in combination with opioids, for moderate to severe pain. The commonly used NSAIDs for cancer pain include ibuprofen, diclofenac, acetaminophen, indomethacin, and celecoxib.
The use of of NSAIDs has been associated with peptic ulcers, gastrointestinal bleeding, platelet dysfunction, renal injury, and liver injury. The incidences of the adverse reactions are associated with the dosage and duration of NSAIDs. The defined daily doses (DDD) of NSAIDs are as follows: Ibuprofen 2,400 mg/d, acetaminophen 2,000 mg/d, and celecoxib 400 mg/d. The NSAIDs display a “ceiling” effect, i.e. increasing the dose above a given level does not provide further relief; instead, it may remarkably increase the drug toxicity. Therefore, if long-term use of an NSAID is required, or, when the DDD of an NSAID has already reached, an opioid should be used instead. Or, in a combination therapy, only the dose of an opioid should be increased.
- (ii) Opioid analgesics. Opioid analgesics are the treatment of choice for moderate and severe pain. Currently, the commonly used short-acting opioid for cancer pain is morphine immediate-release tablets, whereas the long-acting opioids include morphine sustained-release tablets, oxycodone sustained-release tablets, and fentanyl transdermal patches. Opioid receptor agonists are recommended for chronic pain. Oral administration is preferred for long-term opioid use. For patients with clear indications, transdermal patches or temporary subcutaneous injection can be applied; also, patient-controlled analgesia can be scheduled when indicated.
- a) Initial dose titration. The effectiveness and safety of opioids differ among different patients. The process of adjusting their dose until the desired action is obtained is called dose titration. For patients who use an opioid for the first time, titration is conducted based on the following principles: Treat the patient with morphine immediate-release tablets firstly; based on the degree of pain, determine the initial fixed dose as 5 mg -15 mg, Q4h; if no response (or satisfactory response) is achieved, titration should be performed according to the pain intensity one hour later (Table 1), during which the pain intensity and adverse reactions should be closely monitored. After the initial dose, calculate the dosage for the second day: the next day total requirement = previous 24 hour total requirement + the day before yesterday total requirement. On the second day, divide the calculated total dose requirement into 6 oral doses, with the titration doses being 10-20% of the previous 24 hour total requirement. Accordingly, the doses are adjusted on a daily basis until there are stable pain scores below 3. In case that uncontrollable adverse reactions occur in a patient with pain score below 4, the the titration dose should be further reduced by 25%, and meanwhile the disease re-evaluated.

Full table
For opioid-naive patients with moderate to severe cancer pain, short-acting formulations are recommended for the initial medication. Dose titration should be performed individually. When a desired dose level (effective and safe) is achieved, long-acting opioid analgesics at the equivalent dose may be applied.
For patients have already used opioids for their pain, the dose should be adjusted according to Table 1.
For patients with stable diseases, the opioid controlled-release tablets may be used as the background drug, upon which opioid short-acting drugs are used as alternatives for breakthrough pain.
- b) Maintenance medication. The commonly used long-acting opioids for aintenance medication in China include morphine sustained-release tablets, oxycodone sustained-release tablets, and fentanyl transdermal patches. During the use of long-acting opioids, short-acting opioid analgesics should be prepared as alternatives. When the disease condition changes, the dose of a long-acting analgesic drug is inadequate, or breakthrough pain occurs, a short-acting opioid should be immediately given for salvage therapy and/or dose titration. Rescue doses are equal to 10-20% of the 24-hour rate. If more than three times of salvage treatment using a short-acting opioid is applied within 24 hours, the previous 24 hour salvage doses should be converted into those of a long-acting opioid per hour.
The dosage conversions among opioids are listed in Table 2. Still, the disease condition should be carefully monitored and the dose should be adjusted individually when shifting to another opioid.

Full table
A plan for tapering opioid therapy should be established: that is, reduce the dose by 30% firstly, then reduce 25% two days later, until the daily dosage is equivalent to 30-mg oral morphine. The drug can be safely stopped after another two days of medication.
- c) Management of adverse reactions. The main adverse reactions of opioids include constipation, nausea, vomiting, drowsiness, itching, dizziness, urinary retention, delirium, cognitive impairment, and respiratory depression. Except for constipation, most adverse reactions are transient or tolerable. The prevention and treatment of adverse reactions of opioid analgesics should be an important part of pain management plan. Nausea, vomiting, drowsiness, dizziness, and some other adverse reactions often occur during the first few days of treatment in opioid-naive patients. For these patients, antiemetics such as metoclopramide may be concurrently administered within the first few days to prevent vomiting and nausea; in patients without nausea, however, antiemetics are not required. Constipation usually occurs throughout opioid treatment, and most patients need to use laxatives to prevent constipation. In patients who experience excessive sedation and/or mental disorders during the treatment, the opioid dose should be reduced. In addition, the potential impacts of renal insufficiency, hypercalcemia, metabolic abnormalities, and psychotropic co-morbidities should be carefully monitored.
- (iii) Adjuvant drugs. The adjuvant drugs for analgesic treatment include anticonvulsants, antidepressants, corticosteroids, N-methyl-D-aspartate receptor (NMDA) antagonist, and tropical anesthetics. The adjuvant drugs can enhance the analgesic effect of opioids or exert direct analgesic effects. The adjuvant analgesics are often used for the adjuvant treatment of neuropathic pain, bone pain, or visceral pain. The selection and dose adjustment of adjuvant drugs should also be individualized. The commonly used adjuvant drugs for neuropathic pain include:
- a) Anticonvulsants (e.g., carbamazepine, gabapentin, and pregabalin): for tearing pain, electric pain, or burning pain due to nerve injury. Gabapentin, orally administered, starting at 100-300 mg once daily, then increase to 300-600 mg three times daily, with the peak dosage of 3,600 mg/d; Pregabalin, 75-150 mg, 2-3 times daily, with the maximum dose of 600 mg/d.
- b) Tricyclic antidepressants (e.g., amitriptyline, duloxetine, and venlafaxine): for numbness and burning pain caused by the central or peripheral nerve injury. These drugs are also helpful to improve mood and/or sleep. Amitriptyline, 12.5-25 mg orally administered, once per night, and gradually increased to the optimal therapeutic dose. During the analgesic medication, the changes in pain scores and adverse drug reactions should be recorded in the medical records to ensure that the cancer pain can be relieved in a safe, effective, and sustained manner.
4.2.3 Non-pharmaceutical therapies
The non-pharmaceutical therapies for cancer pain include physical therapies (e.g., interventional therapy, acupuncture, transcutaneous electrical acupoint stimulation), cognitive-behavioral training, and psychosocial support. The appropriate application of non-pharmaceutical therapies can serve as a useful supplement for the pharmaceutical therapies; when combined with the analgesics, they can enhance the effectiveness of analgesic treatment.
The interventional therapies include nerve block, neurolysis, percutaneous vertebroplasty, neurolytic therapy, nerve stimulation therapy, and radiofrequency ablation. Epidural, spinal, and plexus routes of administration can effectively control cancer pain via single nerve blockage and thus alleviate the gastrointestinal reactions of opioids and reduce their dosages. The adoption of an interventional treatment should be based on the results of a comprehensive assessment of the patient’s expected survival and physical performance, the indications of an anti-tumor therapy, and the potential benefits and risks of the interventional treatment.
5. Patient and family education
The understanding and cooperation from the patients and their families are critical for the success of cancer pain management. Therefore, active patient and family education programs should be provided, which should include: (I) the patient should actively describe their pain to the medical staff; (II) pain management is an important part of multidisciplinary cancer treatment, and it is unnecessary and even harmful to endure pain; (III) most cancer pain can be effectively controlled through drug therapy. During the analgesic treatment, the patient should follow the physician’s instructions and take the drugs regularly. It is unwise to adjust the dosage or change the therapeutic program without consulting the doctor; (IV) morphines are the commonly used drugs for cancer pain treatment. Morphine addiction is rare during the treatment; (V) the drugs should be kept in a safe place; (VI) during the analgesic treatment, the efficacy and adverse reactions of drugs should be closely observed. Communicate with the medical staff timely to facilitate the adjustment of the treatment goals and measures; and (VII) it is very important to go to all of the follow-up appointments.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Annex
Brief Pain Inventory (BPI)
Name: __________________ Case No.: ____________ Diagnosis: _______________________
Date of assessment: _____________________ Physician: _______________________
1. Throughout our lives, most of us have had pain from time to time (such as minorheadaches, sprains, and toothaches). Have you had pain other than these every-day kinds of pain today?
(1) Yes (2) No
2. On the diagram, shade in the areas where you feel pain. Put an X on the area that hurts the most.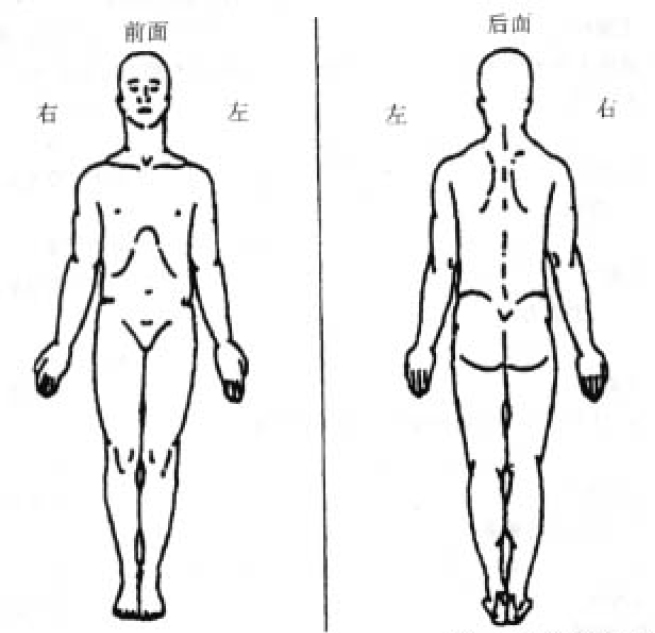
3. Please rate your pain by circling the one number that best describes your pain at its worst in the last 24 hours.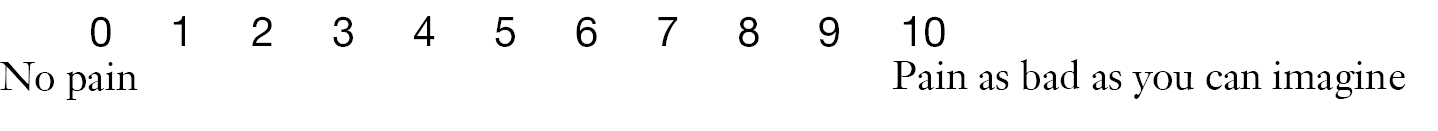
4. Please rate your pain by circling the one number that best describes your pain at its least in the last 24 hours.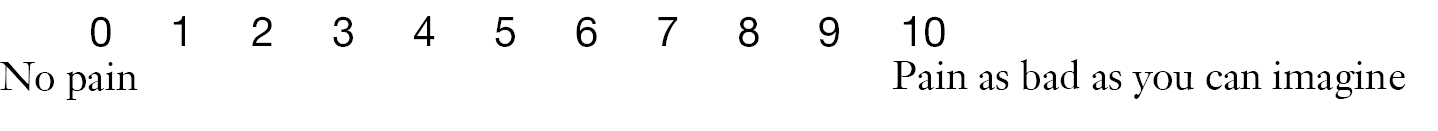
5. Please rate your pain by circling the one number that best describes your pain on the average.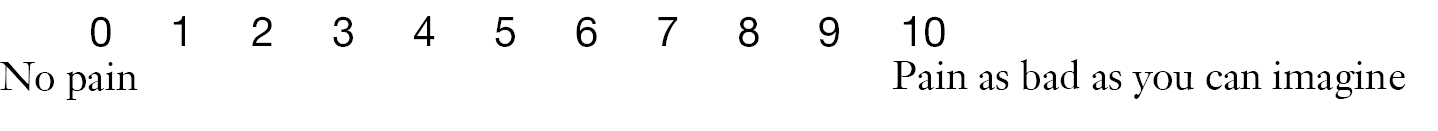
6. Please rate your pain by circling the one number that tells how much pain you have right now.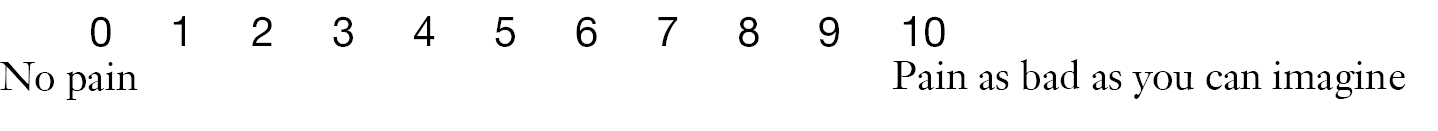
7. What treatments or medications are you receiving for your pain?
—————————————————————————————————
8. In the last 24 hours, how much relief have pain treatments or medications provided? Please circle the one percentage that most shows how much relief you have received.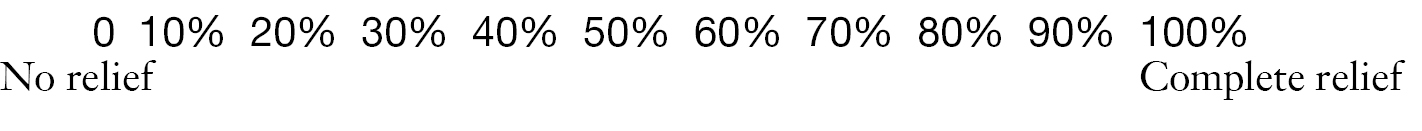
9. Circle the one number that describes how, during the past 24 hours, pain has interfered with your:


