Challenging equipotency calculation for hydromorphone after long-term intravenous application
Introduction
Pain is a serious problem occurring in up to 75% of cancer patients. The World Health Organization (WHO) has set up a three step approach in the treatment of cancer pain, which provides sufficient pain control for the majority of patients (1). However, despite these guidelines, up to 30% of patients do not receive adequate pain relief (2). Particularly after long-term opioid medication, this phenomenon is visible. Opioid rotation is a common practice, which is effective in up to 50-80% of patients (3). Especially, parenteral administration of opioids is useful for rapid titration in patients with severe pain, requiring doses no longer convenient for transdermal or oral applications. Even though equianalgesic dose tables exist, it is recognized that individual patients vary greatly in their response to different opioids (4). This report demonstrates a case of successful symptom management through opioid rotation at a dose far less than the equivalent dose (1/9) based on standard conversion table. It impressively emphasizes the need of new opioid titration after long-term opioid application.
Case presentation
A 60-year-old woman with a history of inoperable urothelial cell carcinoma (T4bN3M0G3) was presented to our Department of Pain Medicine. Following radio-chemotherapy and ureteral stent implantations, she developed increasing pain over a period of two years. Medical history revealed that her pain medication started with WHO I (dipyrone) followed by WHO II (dipyrone and tramadol) and WHO III, beginning with fentanyl TTS. As a result of insufficient pain control, fentanyl TTS was rotated to transdermal buprenorphine in the course of time. The patient reported that even dosage escalation up to 140 µg/h buprenorphine did not provide sufficient analgesia. Therefore, titrations with levomethadone and morphine were performed. However, both medications had to be stopped because of hereditary side effects. The patient finally received a patient-controlled analgesia (PCA) at home with hydromorphone, which initially provided satisfactory analgesia. The dosage subsequently escalated up to 120 mg per day. During her first visit at our Clinic, she rated continuous pain of 9/10 on a numeric rating scale (NRS 0= no pain, 10= worst pain imaginable), compounded by episodes of increased intensity (NRS 10/10). The quality of pain was dull and throbbing. Her current pain medication was hydromorphone via intravenous PCA (Hydromorphone 2 mg/mL, continuous rate 1.4 mg/h, bolus 3 mg, interval lock 60 min: average dose 100 mg/24 h) and dipyrone (500 mg, 1-1-1-1). She also received pregabaline 150 mg 1-0-1 due to chemotherapy-induced polyneuropathy.
Case management
After one single intravenous bolus of 7.5 mg piritramide, an opioid with a potency of 0.75 as compared to morphine, the patient reported a decrease of pain intensity from NRS 9/10 to NRS 2/10, with occasional peaks of NRS 3. Due to this unexpectedly good response, we supplemented her current medication (hydromorphone) with a piritramide-PCA (2.5 mg piritramide/mL, no continuous application, bolus 2.5 mg, interval lock 15 min, dose limit 30 mg/4 h). Running basal rate of hydromorphone was continued, but patient-initiated bolus application of hydromorphone was stopped. Consequently, we switched the patient from intravenous hydromorphone to intravenous piritramide within one week through a stepwise reduction of hydromorphone basal rate and stepwise elevation of piritramide basal rate. Due to this cautiously performed overlapping rotation between the two opioids, the patient did not show any signs of abstinence or withdrawal. The medication with dipyrone and pregabaline was continued. Table 1 chronologically presents all the opioids used including their specific pharmacological properties (Table 2) as well as their equipotent dosages compared to morphine, demonstrating a surprisingly low piritramide requirement.
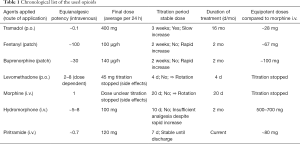
Full table
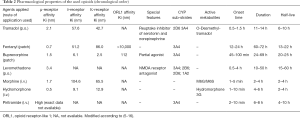
Full table
Case outcome
The patient was reassessed daily to determine response to therapy and tolerability of the new opioid. After 7 days of inpatient stepwise rotation, hydromorphone was completely discontinued and the patient received an average daily piritramide dose of 120 mg, equivalent to approximately 14 mg of intravenous hydromorphone, based on standard conversion tables. PCA-settings at the end of titration were: 3 mg piritramide/ml, basal rate 2.0 mg/h, bolus 3 mg, interval lock 30 min. On discharge two days later, stable satisfactory analgesia of NRS 1 at rest and NRS 2 during movement was maintained with this opioid medication as well as dipyrone and pregabaline.
Discussion
Opioid rotation is an important, but challenging aspect of pain treatment. When switching a patient from one opioid to another, knowledge of the respective conversion ratio is crucial. However, conversion tables are not based on well-designed studies and do not include special pharmacodynamics and pharmacokinetic properties of the respective opioids (2-4,17).
Our patient was successfully switched to piritramide after long-term intravenous use of hydromorphone. Given an accepted equipotency ratio of 5‒7:1 for hydromorphone: morphine and a ratio of 0.7:1 for piritramide: morphine, the calculated daily dose of piritramide would have been more than seven times higher than the dose required by our patient. Since breakthrough pain is usually treated by 1/5‒1/6 of the daily opioid dose, our patient would have received a bolus of nearly 150 mg piritramide instead of the given single dose of 7.5 mg (18). In accordance with our report, frequent cases of inconsistencies in opioid equianalgesic ratios with even life threatening events are described (4). There are great differences in the pharmacological properties of opioids and the response to opioids differs from person to person. It is influenced by multiple factors including gender, age, race, level of education, and genetic polymorphisms (19).
Piritramide (1-(3-cyano-3.3-diphenyl-propyl)-4-(1-piperidyl)piperidine-4-carboxamide) is a synthetic full mu receptor agonist with an equipotency ratio to morphine of about 0.65‒0.75 (5,6). It is only available for intravenous, subcutaneous and intramuscular application and is, therefore, commonly used for perioperative pain treatment in European countries, often via PCA. Due to its piperidino ring, piritramide has an uncommon structure for an opioid. The onset of action is within 2‒10 min and its terminal elimination half-life is about 8h. Piritramide has a large mean volume of distribution of approximately 4.7 litre kg−1 and a relatively long equilibration half-life between plasma and the effect site of approximately 16.8 min. It is mainly eliminated by hepatic metabolism (6,20).
Hydromorphone is a strong opioid which is widely used particularly in cancer pain management. Opioids are substantially metabolized by cytochrome P450 (CYP450) enzymes and to a lesser extent by UDP-glucuronosyltransferases (UGT). A large number of CYP450 enzymes have been discovered so far. Among them, CYP1A2, CYP2C9, CYP2D6, CYP3A4, and CYP3A5 enzymes are involved in the metabolism of the majority of drugs undergoing this type of biotransformation (7). A genetic polymorphism in the CYP450 enzymes may account for different concentrations of either the administered opioid or its active metabolites at the site of action (8). While piritramide is metabolized via CYP3A4, hydromorphone is metabolized via UGT (7,9). Our patient did not receive any medication, which might have interacted with CYP3A4 or UGT. However, since we did not perform genetic analyses, we cannot rule out whether genetic polymorphism might explain the different responses to piritramide and hydromorphone.
Conclusions
Cancer pain management is a challenging topic for clinicians. In some cases, an unusual opioid rotation from a more potent opioid to a less potent may be helpful due to different pharmacological properties. Our report emphasizes the need of opioid titration in order to provide individually tailored pain medicine for cancer pain management.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient’s husband for publication of this manuscript and any accompanying images.
References
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65-76. [Crossref] [PubMed]
- Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med 2011;25:504-15. [Crossref] [PubMed]
- Mercadante S, Bruera E. Opioid switching: a systematic and critical review. Cancer Treat Rev 2006;32:304-15. [Crossref] [PubMed]
- Webster LR, Fine PG. Review and critique of opioid rotation practices and associated risks of toxicity. Pain Med 2012;13:562-70. [Crossref] [PubMed]
- Lehmann KA, Tenbuhs B, Hoeckle W. Patient-controlled analgesia with piritramid for the treatment of postoperative pain. Acta Anaesthesiol Belg 1986;37:247-57. [PubMed]
- Kumar N, Rowbotham DJ. Piritramide. Br J Anaesth 1999;82:3-5. [Crossref] [PubMed]
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005;352:2211-21. [Crossref] [PubMed]
- Somogyi AA, Barratt DT, Coller JK. Pharmacogenetics of opioids. Clin Pharmacol Ther 2007;81:429-44. [Crossref] [PubMed]
- Pergolizzi J, Böger RH, Budd K. Det al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008;8:287-313. [Crossref] [PubMed]
- Bartošová O, Polanecky O, Perlik F. Aet al. OPRM1 and ABCB1 polymorphisms and their effect on postoperative pain relief with piritramide. Physiol Res 2015;64 Suppl 4:S521-7. [PubMed]
- Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic. J Pharmacol Exp Ther 1992;260:275-85. [PubMed]
- Drewes AM, Jensen RD, Nielsen LM, et al. Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol 2013;75:60-78. [Crossref] [PubMed]
- Zaveri N, Polgar WE, Olsen CM, et al. Characterization of opiates, neuroleptics, and synthetic analogs at ORL1 and opioid receptors. Eur J Pharmacol 2001;428:29-36. [Crossref] [PubMed]
- Emmerson PJ, Liu MR, Woods JH, et al. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 1994;271:1630-7. [PubMed]
- Trescot AM, Datta S, Lee M, et al. Opioid pharmacology. Pain Physician 2008;11:S133-53. [PubMed]
- Karow T, Lang-Roth R. Allgemeine und Spezielle Pharmakologie und Toxikologie, 2016:581.
- Devarakonda K, Vandenbossche J, Richarz U. Complementary pharmacokinetic measures to further define the profile of once-daily OROS hydromorphone ER during single-dose and steady-state dosing. Springerplus 2013;2:625. [Crossref] [PubMed]
- Mercadante S, Villari P, Ferrera P, et al. Safety and effectiveness of intravenous morphine for episodic (breakthrough) pain using a fixed ratio with the oral daily morphine dose. J Pain Symptom Manage 2004;27:352-9. [Crossref] [PubMed]
- Angst MS, Phillips NG, Drover DR, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain 2012;153:1397-409. [Crossref] [PubMed]
- Janssen PA. Pirinitramide (R 3365), a potent analgesic with unusual chemical structure. J Pharm Pharmacol. 1961;13:513-30. [Crossref] [PubMed]


