Editor’s note: “Palliative Radiotherapy Column” features articles emphasizing the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L. W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles and perspectives relating to palliative radiotherapy, editorials and commentaries on recently published trials and studies.
Assessment of quality of life in phase III trials of radiotherapy in localized or locally advanced head and neck cancer over the past 17 years
Introduction
Head and neck cancer is among the most common tumor types worldwide, with 543,941 new cases and 228,729 deaths from lip, oral cavity, larynx and nasopharynx cancers estimated for 2012 (1). In the United States, oral cavity and pharynx tumors account for 2.4% of all malignancies, with approximately 49 thousand new cases and 9.5 thousand deaths estimated every year (2). Standard therapy for head and neck cancer has evolved considerably over the past 15 years, with major advances in terms of our ability to provide local control, organ preservation and improvement of survival through the use of combined-modality therapy and targeted agents (3-12). However, depending on the stage, primary site and pattern of spread, head and neck tumors can cause various degrees of structural deformities and functional handicaps, compromising patient comfort, social integration, and quality of life (QOL) (13,14). Moreover, treatment for head and neck cancer can induce mutilation and further compromise patient functioning, thus worsening QOL.
Over the past years, health care has gradually broadened its effort to comprise patient well-being and health-related quality of life (HRQOL) as essential outcomes, especially in medical oncology (15). The primary aim of randomized controlled trials is usually to assess the efficacy of interventions through the use of endpoints such as objective response rates, disease-free or progression-free survival, and overall survival (OS). Nevertheless, progressively more attention has been paid to improving the way patients live during cancer treatment (16,17). Although HRQOL evaluation has several potential values and repercussions for research and for clinical practice, the role of HRQOL data to support the selection of therapy for head and neck cancer is still unclear. In the current study, we sought to investigate the extent to which QOL parameters have been used in recent studies on head and neck cancer, as well as the frequency and correlates of significant QOL gains.
Methods
Search strategy
We used the medical subject headings ‘head and neck neoplasms’ and ‘radiotherapy’ to search PubMed for the main paper reporting phase III trials published in English language between 1/1999 and 12/2015 in 16 leading journals that publish results of most of the clinical trials in this field (Annals of Oncology, Archives of Otolaryngology-Head & Neck Surgery, British Journal of Cancer, Cancer, Clinical Cancer Research, European Journal of Cancer, Head & Neck, International Journal of Radiation Oncology, Biology, and Physics, Journal of Clinical Oncology, Journal of the National Cancer Institute, Oral Oncology, Radiotherapy & Oncology, The Lancet, The Lancet Oncology, The Laryngoscope, and The New England Journal of Medicine).
We focused on studies for which radiation therapy had been an important component of treatment in at least one of the arms. We excluded papers reporting exclusively on esophageal cancer, those investigating topical or surgical therapies as the main research variable, those in which patients were randomized after completion of the main therapy, those on retreatment or reporting combined analysis of trials already selected for analysis, those on preliminary or long-term results of trials whose main paper was already selected (when the main paper was not in the 17-year period chosen for analysis, the preliminary or long-term-results paper was kept), and those on correlative biology or prognostic factors in isolation from the main trial results. We excluded randomized trials with no stated phase in the title or abstract if the number of evaluable patients per arm was <100. Since our objective was to investigate the results of studies that are likely to impact clinical practice given their publication in broadly read periodicals, no effort was made to control for publication bias.
Collection of HRQOL data
For each study identified, we abstracted the overall features of the trial (such as number of patients and arms, along with treatment type and line) and data on the use of endpoints, including HRQOL parameters. Regarding HRQOL as an endpoint in the trials, we first attempted to recognize any mention in the article of HRQOL data collection during the study, or, when no such information was available, the presence of a companion article with HRQOL analysis independently. When HRQOL was a trial endpoint, we collected data on the instruments used for HRQOL analysis, evaluating whether there was formal statistical comparison between study arms and the results of such comparisons as informed by the authors of the article. Lastly, we evaluated whether the HRQOL analysis was cited in the abstract of the articles.
Statistical analysis
Fisher’s exact test and the Mann-Whitney test, considering a two-sided significance level of 5%, were used to compare categorical and continuous variables between groups of studies, respectively.
Results
Characteristics of the studies
Our search identified 88 phase III trials that were eligible for analysis, and their most relevant characteristics are displayed in Table 1. Such studies enrolled a total of 32,707 evaluable patients in 191 trial arms. The median number of evaluable patients per study was 263 (range, 58 to 1,485), and the median number of patients per arm was 144 (range, 30 to 743). Twenty-six studies included patients with nasopharyngeal carcinoma, in 20 cases in an exclusive fashion. The primary endpoint was related to local control in 40 trials and to OS in 27 cases; for other trials, miscellaneous primary or co-primary endpoints were used. Of note, HRQOL was never used as primary endpoint.

Full table
Analysis of HRQOL
The assessment of HRQOL was reportedly performed in 20 trials (22.7%), whose main features are shown in Table 2 (5,9,18-35). For these trials, HRQOL was always a secondary endpoint, and the primary endpoint was related to local or locoregional control in seven trials and to OS in four trials. There was no significant difference in the median sample size of studies with or without HRQOL assessment (344 vs. 298 patients; P=0.400). Also, there was no statistically significant trend for reporting of HRQOL when studies from the first 9 years (20.4%) were compared with those from the second 8 years, considering date of publication (25.6%; P=0.614).
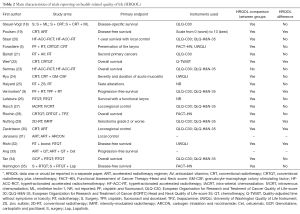
Full table
Comparisons of HRQOL parameters within a trial were reported in 17 of 20 trials. Statistically significant differences between such parameters were reportedly found in only six studies, four of which favoring the experimental arm. Given the low number of studies with significant QOL differences, we explored no correlates of such finding.
Discussion
The severity of problems associated with head and neck cancer and its treatment is related to the therapeutic modalities used and the anatomic site and extent of the disease (36). Since the majority of patients are diagnosed with locally advanced disease, treatment tends to be aggressive, with potential for significant acute and long-term adverse effects. The assessment of HRQOL has become an important component of clinical cancer research, and HRQOL endpoints have gained increased use in clinical trials (17). There are several instruments that aim at analyzing HRQOL specifically in patients with head and neck cancer (37-42). The use of validated HRQOL instruments may allow a better understanding of the toxicity of head and neck cancer treatment. Moreover, the assessment of HRQOL in cancer patients may theoretically facilitate selection among different treatment choices, and even serve as a prognostic.
Several authors have investigated the relationship between HRQOL and regional control or OS in patients with head and neck cancer (43-46). Investigators from the Radiation Therapy Oncology Group (RTOG) have analyzed prospectively collected HRQOL data from patients enrolled in two RTOG randomized phase III trials to assess their value as an independent prognostic factor for locoregional control and/or OS (45). Baseline Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N) were found on multivariate analysis to independently predict locoregional control but not OS. Meyer et al. have conducted a study of 540 patients, using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and Head and Neck Radiotherapy Questionnaire (46). That study suggested that the baseline physical functioning score was an independent predictor of OS among patients with localized head and neck cancer treaded with radiation therapy, and similar results have been reported by Fang et al. (43) and Karvonen-Gutierrez et al. (44).
Despite the possible benefits of measuring HRQOL scores in patients with head and neck cancer, the results of non-randomized studies and retrospective analyses must be interpreted cautiously. The current study shows that HRQOL endpoints have been used in approximately 21% of contemporary phase III trials in head and neck cancer. In most of the trials in which such endpoints were used, formal HRQOL statistical comparisons between groups were undertaken, but significant differences between groups were found in only three of 12 trials. Our study suffers from limitations, the most important of which being the fact that publication bias was not controlled for, as we have only analyzed papers published within a limited period of time in selected medical journals. By using this study design, we could not ascertain whether unpublished studies have used HRQOL endpoints or found statistically significant differences between groups with different rates than those reported herein. A second important limitation of the current work is the joint analysis of all types of HRQOL assessment, regardless of the instruments used or HRQOL domains analyzed, as this type of analysis was beyond our scope. It is possible that a more in-depth evaluation of HRQOL results in head and neck cancer might provide important information regarding the differential performance of specific HRQOL instruments or modes of analysis.
These limitations notwithstanding, we believe our study design allows for an overview of contemporary practice in HRQOL research in head and neck cancer, since the journals analyzed currently publish most of the randomized clinical trials in this setting. As a result, we believe our results indicate that HRQOL has been infrequently investigated in phase III trials of radiation therapy in head and neck cancer. In breast cancer, for example, a study using a similar methodology has found that HRQOL was assessed in 40% of recent phase III trials in breast cancer (47). On the other hand, only a minority of the phase III trials in breast cancer demonstrated a significant difference between groups, a result very similar to those reported in the current study. Thus, both of these studies suggest that although HRQOL is one of the key indicators of treatment benefit in oncology, contemporary systemic therapies do not appear to affect HRQOL differentially. While the reasons for this latter finding are uncertain, it appears that more efforts are needed in order to understand the role of HRQOL assessment in phase III trials of radiotherapy for head and neck cancer.
Acknowledgments
The authors would like to thank Rita de Cassia Ortega Borges and Adriana Mara Fonseca for providing literature search assistance.
Footnote
Conflicts of Interest: Identification of meetings at which the manuscript was presented at ASCO Annual Meeting, 2011, Chicago. J Clin Oncol 2011;29 suppl:e19524.
References
- World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: (Accessed 15/07/2016).http://globocan.iarc.fr/Default.aspx
- Buyse M, Saad ED, Burzykowski T. Adaptive Randomization of Neratinib in Early Breast Cancer. N Engl J Med 2016;375:1591-4. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [PubMed]
- Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92-8. [Crossref] [PubMed]
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. [Crossref] [PubMed]
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945-52. [Crossref] [PubMed]
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937-44. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [Crossref] [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [Crossref] [PubMed]
- Mendez LC, Moraes FY, Poon I, et al. The management of head and neck tumors with high technology radiation therapy. Expert Rev Anticancer Ther 2016;16:99-110. [Crossref] [PubMed]
- Marta GN, Riera R, Bossi P, et al. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: Systematic review and meta-analysis. Eur J Cancer 2015;51:2596-603. [Crossref] [PubMed]
- Marta GN, William WN Jr, Feher O, et al. Induction chemotherapy for oral cavity cancer patients: Current status and future perspectives. Oral Oncol 2015;51:1069-75. [Crossref] [PubMed]
- Agarwal SK, Munjal M, Koul R, et al. Prospective evaluation of the quality of life of oral tongue cancer patients before and after the treatment. Ann Palliat Med 2014;3:238-43. [PubMed]
- Agarwal SK, Gogia S, Agarwal A, et al. Assessment of voice related quality of life and its correlation with socioeconomic status after total laryngectomy. Ann Palliat Med 2015;4:169-75. [PubMed]
- Michael M, Tannock IF. Measuring health-related quality of life in clinical trials that evaluate the role of chemotherapy in cancer treatment. CMAJ 1998;158:1727-34. [PubMed]
- Minasian LM, O'Mara AM, Reeve BB, et al. Health-related quality of life and symptom management research sponsored by the National Cancer Institute. J Clin Oncol 2007;25:5128-32. [Crossref] [PubMed]
- Bottomley A, Aaronson NK, European Organisation for Research and Treatment of Cancer. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol 2007;25:5082-6. [Crossref] [PubMed]
- Steuer-Vogt MK, Bonkowsky V, Ambrosch P, et al. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: a randomised controlled clinical trial. Eur J Cancer 2001;37:23-31. [Crossref] [PubMed]
- Poulsen MG, Denham JW, Peters LJ, et al. A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans-Tasman Radiation Oncology Group Study. Radiother Oncol 2001;60:113-22. [Crossref] [PubMed]
- Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy--results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;50:1161-71. [Crossref] [PubMed]
- Bairati I, Meyer F, Gélinas M, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst 2005;97:481-8. [Crossref] [PubMed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Semrau R, Mueller RP, Stuetzer H, et al. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;64:1308-16. [Crossref] [PubMed]
- Ryu JK, Swann S, LeVeque F, et al. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys 2007;67:643-50. [Crossref] [PubMed]
- Halyard MY, Jatoi A, Sloan JA, et al. Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? Results of phase III double-blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4). Int J Radiat Oncol Biol Phys 2007;67:1318-22. [Crossref] [PubMed]
- Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst 2009;101:142-52. [Crossref] [PubMed]
- Rasch CR, Hauptmann M, Schornagel J, et al. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 2010;116:2159-65. [Crossref] [PubMed]
- Rischin D, Peters LJ, O'Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010;28:2989-95. [Crossref] [PubMed]
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127-36. [Crossref] [PubMed]
- Zackrisson B, Nilsson P, Kjellén E, et al. Two-year results from a Swedish study on conventional versus accelerated radiotherapy in head and neck squamous cell carcinoma--the ARTSCAN study. Radiother Oncol 2011;100:41-8. [Crossref] [PubMed]
- Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012;30:1777-83. [Crossref] [PubMed]
- Rishi A, Ghoshal S, Verma R, et al. Comparison of concomitant boost radiotherapy against concurrent chemoradiation in locally advanced oropharyngeal cancers: a phase III randomised trial. Radiother Oncol 2013;107:317-24. [Crossref] [PubMed]
- Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014;32:2940-50. [Crossref] [PubMed]
- Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952-60. [Crossref] [PubMed]
- Harrington K, Temam S, Mehanna H, et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients With Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Oncol 2015;33:4202-9. [Crossref] [PubMed]
- Marta GN, Silva V, de Andrade Carvalho H, et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol 2014;110:9-15. [Crossref] [PubMed]
- List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer 1990;66:564-9. [Crossref] [PubMed]
- Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol 1992;31:311-21. [Crossref] [PubMed]
- Browman GP, Levine MN, Hodson DI, et al. The Head and Neck Radiotherapy Questionnaire: a morbidity/quality-of-life instrument for clinical trials of radiation therapy in locally advanced head and neck cancer. J Clin Oncol 1993;11:863-72. [PubMed]
- List MA, D'Antonio LL, Cella DF, et al. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer 1996;77:2294-301. [Crossref] [PubMed]
- Trotti A, Johnson DJ, Gwede C, et al. Development of a head and neck companion module for the quality of life-radiation therapy instrument (QOL-RTI). Int J Radiat Oncol Biol Phys 1998;42:257-61. [Crossref] [PubMed]
- Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001;127:870-6. [PubMed]
- Fang FM, Liu YT, Tang Y, et al. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 2004;100:425-32. [Crossref] [PubMed]
- Karvonen-Gutierrez CA, Ronis DL, Fowler KE, et al. Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol 2008;26:2754-60. [Crossref] [PubMed]
- Siddiqui F, Pajak TF, Watkins-Bruner D, et al. Pretreatment quality of life predicts for locoregional control in head and neck cancer patients: a radiation therapy oncology group analysis. Int J Radiat Oncol Biol Phys 2008;70:353-60. [Crossref] [PubMed]
- Meyer F, Fortin A, Gélinas M, et al. Health-related quality of life as a survival predictor for patients with localized head and neck cancer treated with radiation therapy. J Clin Oncol 2009;27:2970-6. [Crossref] [PubMed]
- Adamowicz K, Jassem J, Katz A, et al. Assessment of quality of life in advanced breast cancer. An overview of randomized phase III trials. Cancer Treat Rev 2012;38:554-8. [Crossref] [PubMed]


