Editor’s note:
“Palliative Radiotherapy Column” features articles emphasizing the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L. W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles and perspectives relating to palliative radiotherapy, editorials and commentaries on recently published trials and studies.
Palliative radiotherapy for skin malignancies
Introduction
Skin cancer is the most prevalent cancer in the United States (1,2). Early intervention may even be curative for patients with either basal cell carcinoma (BCC) or squamous cell carcinoma (SCC) subtypes, which respectively constitute approximately 70–80% and 20% of non-melanoma skin cancers (NMSC) (3,4). Additionally, early detection of melanoma and surgical excision with Mohs micrographic surgery (MMS) can achieve a 5-year overall survival of 97% in stage I patients, while the rate markedly decreases to 15–20% in those with distant metastatic disease (5). Despite advances in new treatments for melanoma, it still accounts for 70–90% of all skin cancer-related deaths (3,6).
Palliative care, as defined by the World Health Organization, includes improving patient quality of life (QoL) and relieving symptomatic distress (7). In the context of skin cancer, palliative care may address many patient concerns including physical tumor-related pain, psychosocial distress from being in public with visible disease, increased time spent in a health-care environment for care, and financial costs (8-13). It is with these factors in mind that treatment selection for advanced skin malignancies must incorporate patient goals and preferences.
The focal point of the localized treatment landscape in skin cancer revolves around surgical intervention. For BCC and SCC, the primary local therapies include MMS, cryotherapy, radiation therapy (RT), and curettage and electrodissection (C&E) (14,15). Other therapies such as imiquimod, cryosurgery, or photodynamic therapy only target the superficial skin and are indicated for patients who choose not to undergo surgery or radiation. For melanoma, surgical excision is also the mainstay for primary localized melanoma, however topical imiquimod or radiation may be used as an alternative depending on tumor location and patient comorbidities (16). In the rarer NMSCs such as Merkel cell carcinoma (MCC) and dermatofibrosarcoma protuberans (DFSP), surgical approaches remain the primary mainstay of treatment (17-22). MCC and DFSP are highly-recurrent cancers (median time to recurrence; MCC: 5.5–16.5 months, DFSP: 1–2 years) and often require a wide surgical margin (up to 4 vs. 2 cm in melanoma and 1 cm in BCC/SCC) that may result in disfiguring post-operative scarring (17-19,21). In light of the varying treatment considerations, we herein provide a focused review of the role of RT as a palliative modality in BCC, SCC, and melanoma.
RT with palliative intent is directed towards improving QoL rather than aiming to cure a patient of their advanced disease (23). In particular, radiation may be used to control cancer-related bleeding, pain, ulcerations leading to infections, and neurological dysfunction (24). Palliative radiotherapy (PRT) may be recommended for patients who have poor performance status, inoperable tumors, and who are otherwise poor candidates for more extensive procedures (25). Palliative treatment should be short-duration for those patients who have a poor prognosis, or are unable to travel for multiple treatments (24). High local control rates may also alleviate patient concerns regarding disease recurrence. With regard to skin cancer, the side effects of RT may manifest as subdermal fibrosis, desquamation, erythema, hypopigmentation, epidermal atrophy, and telangiectasia (26). For the purposes of this review, we have preferentially included studies on palliative RT for skin cancer based upon direct mention of cosmetic outcomes, skin-related side effects from RT that may be used as a surrogate for cosmetic outcome, or more hypofractionated schema.
Methods
Studies related to PRT use in skin cancer were independently identified and evaluated by J Lin and W Vuong from existing literature. Authors included studies pertaining to radiotherapy for skin cancer that addressed any of the following criteria in order of importance: (I) mention of palliative outcomes including cosmesis, pain and other cancer-related symptomatic relief, or comfort; (II) mention of skin-related side effects that may be used as a surrogate for cosmetic outcome; (III) use of fewer than 15 fractions for total treatment. Due to the scarcity of research solely focused on palliative results, studies that used total treatment doses thought of as radical treatment but reported palliative outcomes were considered for inclusion. Sample size, total dose, fraction size, number of treatments, local control, and toxicity were obtained from the published articles.
Results
BCC and SCC
Of the studies screened for PRT in BCC and SCC that matched one of the three criteria listed above, nine were selected as representative studies. Sample sizes ranged from 28 to 1,166 patients in various anatomical locations in the head and neck region as well as the extremities. Common fractionation schemes include 3 to 10 fractions with dose per fraction sizes ranging from 7 to 10 Gy (Table 1).
Cosmetic outcomes from EBRT, based upon the presence of telangiectasia, pigment change, and fibrosis, are reported to be generally satisfactory if not excellent in patients with localized disease (>90% with at least a satisfactory outcome) (27-29,31). In general, fractionated approaches have been employed to reduce skin toxicity while maintaining a high treatment dose to the lesion of interest (Table 1) (25,27-31). Early comparisons examined the side effect profile of a large dose at once (20–22.5 Gy in 1 fraction) versus more fractionated schemes including 40–45 Gy over 10 fractions, or 45–50 Gy in 15 fractions (Table 1 for full listing of schema) (27). Despite the heterogeneous treatment regimens, side effects were limited to fewer than 10% of patients (range, 3–9.6%) and were primarily related to telangiectasia, pigmentation, fibrosis, and treatment-field ulceration (Table 1). Side effects were more commonly found in patients with larger tumors (≥5 cm) or that received higher doses per fraction, although local control rates were similar between fractionation schedules. Additionally, fractionation schemes of 30 Gy in 6 fractions, 35 Gy in 5 fractions, and 24 Gy in 3 fractions have been utilized (Table 1) as a balance of minimizing dose-related toxicity through fractionation while not excessively burdening patients with long courses of radiation. Local control rates are generally excellent, ranging from conservative estimates of 93.3–95% in those treated with superficial orthovoltage (X-ray) radiation (27,29,31). One particular series reported that overall local control rates were lower in patients receiving electron beam RT (29).
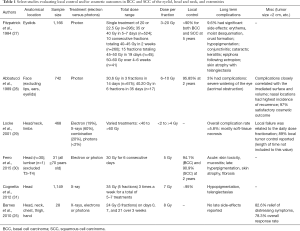
Full table
Direct comparisons of cosmetic outcomes between surgical intervention and RT are rare; however, it has been suggested that surgery may outperform RT regarding long-term cosmetic results (87% vs. 69% “good” evaluation, 4-year follow-up) (32). BCC patients with < 4 cm wide lesions were randomized to surgery or PRT, but it must be noted that only 20 of the RT patients underwent conventional EBRT while other patients had either interstitial brachytherapy or contact therapy (32). More modern evaluations of brachytherapy alone have reported excellent cosmetic outcomes (33). Specifically, an “excellent” response was reported in 94.2% and a “good” response in 3.3% of lesions treated with high-dose-rate electronic brachytherapy based upon the RTOG/EORTC Late Radiation Morbidity Scoring Schema (34).
In addition to cosmesis, PRT may relieve initial presenting symptoms associated with NMSC. Symptoms including pain, bleeding, odor, and/or discharge were successfully palliated in 61.3% of symptomatic lesions or 82.6% of accessible sites by last follow-up using a schedule of 21 Gy in 3 fractions on days 0, 7, and 21 (25). SCCs and BCCs also have a small risk of invading perineural tissue (2.5–14%) which may cause pain (35). Unfortunately, only a small proportion of patients with locally-controlled, perineurally-invasive disease experienced symptomatic relief after PRT. Median RT doses of 70 Gy in 39 fractions obtained a 5-year local control rate of 55% (BCC) and 57% (SCC) in clinical perineural invasion cases, and only 7% of the patients had any symptom relief after successful local control (8).
Melanoma
Ten studies that met one of the three criteria enlisted in the “Methods” section were selected as representative studies. Reports were primarily in the metastatic setting with patient sample sizes ranging from 14 to 466 being treated with total doses ranging from 20 to greater than 60 Gy (Table 2) (36-44). The studies had a variety of anatomic locations treated including cutaneous and lymphatic tissue of the head and neck, thorax, abdominal, and groin regions as well as brain (Table 2).
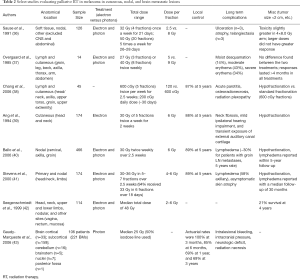
Full table
Melanoma has had a historical reputation for radiation resistance; however, a growing body of clinical evidence has shown that it has a higher repair capacity and is more susceptible to larger doses of radiation per treatment fraction (Table 2). Initially, comparisons of scheduling 32 Gy in 4 fractions versus 50 Gy in 20 fractions demonstrated higher toxicity than in the 32 Gy ×4 fractions arm with equivocal remission rates (Table 2) (36). Notably, patients were randomized without controlling for tumor size or volume. A separate evaluation of 204 lesions demonstrated that complete response rates were significantly associated with fraction size (24% vs. 57% in fractions of < 4 vs. ≥4 Gy respectively, P< 0.001) and that controlling for tumor size resulted in more accurate estimates of total delivered radiation through the extrapolated total dose measure (44). Similarly, patients receiving a total dose greater than 30 Gy have a longer, durable response (7 vs. 4 months for treatments of < 30 Gy) (45). Associated clinical toxicities are similar with PRT use in BCC and SCC for cutaneous lesions and include ulceration, atrophy, telangiectasia, moist desquamation, and erythema (Table 2). However, palliation of nodally-metastatic lesions incurs a risk of lymphedema at rates of 30–58% depending on location (Table 2).
Discussion
Managing malignancies of the skin requires comprehensive QoL evaluation including post-treatment cosmesis and psychosocial stress that must be weighed against clinical outcomes. The majority of skin cancer patients are treated with surgery, although radiation also offers a high degree of tumor control while preserving patient cosmesis. Our review sought to highlight the current evidence for use of PRT in skin cancer including BCC, SCC, and melanoma.
Cosmetic outcomes were generally satisfactory based upon patient perception and objective measures such as skin-related toxicities as reported by the RTOG/EORTC guidelines. In the context of BCC and SCC, the use of varying fractionation schemes did not significantly affect local control although the dose per fraction size influenced toxicity rates. Thus, schema should try to maximize treatment-dose while minimizing toxicity, meaning fewer fractions should be used. This translates to ranges of 24–35 Gy in 3 to 6 fractions. In the setting of melanoma, greater fraction sizes have been associated with better outcomes and schedules utilizing at least 4 Gy per fraction for a total dose greater than 30 Gy are recommended. While symptomatic outcomes vary based upon tumor location, PRT can successfully alleviate pain, bleeding, and neurological symptoms. More extensive research is still necessary to fully evaluate the QoL outcomes associated with PRT, particularly in patients treated with current technologies.
Acknowledgements
The authors would like to thank Angela M. Guerrero and Gefei A. Zhu for consultation regarding the availability of skin cancer care guidelines as provided by the American Academy of Dermatology. Additionally, the authors would like to thank Michael Salvaggio for initial efforts in collecting relevant literature.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Editor’s note: “Palliative Radiotherapy Column” features articles emphasizing the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L. W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles and perspectives relating to palliative radiotherapy, editorials and commentaries on recently published trials and studies.
References
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med 2015;48:183-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf
- Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol 2007;4:462-9. [Crossref] [PubMed]
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline--Update 2012. Eur J Cancer 2012;48:2375-90. [Crossref] [PubMed]
- World Health Organization. WHO Definition of Palliative Care. Available online: http://www.who.int/cancer/palliative/definition/en/
- Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck 2003;25:1027-33. [Crossref] [PubMed]
- Sobanko JF, Sarwer DB, Zvargulis Z, et al. Importance of physical appearance in patients with skin cancer. Dermatol Surg 2015;41:183-8. [Crossref] [PubMed]
- Essers B, Nieman F, Prins M, et al. Perceptions of facial aesthetics in surgical patients with basal cell carcinoma. J Eur Acad Dermatol Venereol 2007;21:1209-14. [PubMed]
- Hawkins DM, Jacobsen G, Johnson CC, et al. Self-reported quality of life after skin cancer in young adults. J Dermatolog Treat 2015;26:357-60. [Crossref] [PubMed]
- Gaulin C, Sebaratnam DF, Fernández-Peñas P. Quality of life in non-melanoma skin cancer. Australas J Dermatol 2015;56:70-6. [Crossref] [PubMed]
- Radiotis G, Roberts N, Czajkowska Z, et al. Nonmelanoma skin cancer: disease-specific quality-of-life concerns and distress. Oncol Nurs Forum 2014;41:57-65. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines: Basal Cell Skin Cancer. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf
- National Comprehensive Cancer Network. NCCN Guidelines: Squamous Cell Skin Cancer. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf
- National Comprehensive Cancer Network. NCCN Guidelines: Melanoma. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000;88:2711-20. [Crossref] [PubMed]
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol 1997;37:600-13. [Crossref] [PubMed]
- Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur J Surg Oncol 2004;30:341-5. [Crossref] [PubMed]
- Kimmel Z, Ratner D, Kim JY, et al. Peripheral excision margins for dermatofibrosarcoma protuberans: a meta-analysis of spatial data. Ann Surg Oncol 2007;14:2113-20. [Crossref] [PubMed]
- Tai P. A practical update of surgical management of merkel cell carcinoma of the skin. ISRN Surg 2013;2013:850797. [Crossref] [PubMed]
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol 2009;16:2985-93. [Crossref] [PubMed]
- Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol 2014;32:2913-9. [Crossref] [PubMed]
- Jones JA, Lutz ST, Chow E, et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin 2014;64:296-310. [Crossref] [PubMed]
- Barnes EA, Breen D, Culleton S, et al. Palliative radiotherapy for non-melanoma skin cancer. Clin Oncol (R Coll Radiol) 2010;22:844-9. [Crossref] [PubMed]
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol 2003;44:159-66. [Crossref] [PubMed]
- Fitzpatrick PJ, Thompson GA, Easterbrook WM, et al. Basal and squamous cell carcinoma of the eyelids and their treatment by radiotherapy. Int J Radiat Oncol Biol Phys 1984;10:449-54. [Crossref] [PubMed]
- Abbatucci JS, Boulier N, Laforge T, et al. Radiation therapy of skin carcinomas: results of a hypofractionated irradiation schedule in 675 cases followed more than 2 years. Radiother Oncol 1989;14:113-9. [Crossref] [PubMed]
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys 2001;51:748-55. [Crossref] [PubMed]
- Ferro M, Deodato F, Macchia G, et al. Short-Course Radiotherapy in Elderly Patients with Early Stage Non-Melanoma Skin Cancer: A Phase II Study. Cancer Invest 2015;33:34-8. [Crossref] [PubMed]
- Cognetta AB, Howard BM, Heaton HP, et al. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol 2012;67:1235-41. [Crossref] [PubMed]
- Petit JY, Avril MF, Margulis A, et al. Evaluation of cosmetic results of a randomized trial comparing surgery and radiotherapy in the treatment of basal cell carcinoma of the face. Plast Reconstr Surg 2000;105:2544-51. [Crossref] [PubMed]
- Paravati AJ, Hawkins PG, Martin AN, et al. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol 2015;5:e659-64. [Crossref] [PubMed]
- RTOG Fundation. RTOG/EORTC Late Radiation Morbidity Scoring Schema. Available online: https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx
- Han A, Ratner D. What is the role of adjuvant radiotherapy in the treatment of cutaneous squamous cell carcinoma with perineural invasion? Cancer 2007;109:1053-9. [Crossref] [PubMed]
- Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys 1991;20:429-32. [Crossref] [PubMed]
- Overgaard J, von der Maase H, Overgaard M. A randomized study comparing two high-dose per fraction radiation schedules in recurrent or metastatic malignant melanoma. Int J Radiat Oncol Biol Phys 1985;11:1837-9. [Crossref] [PubMed]
- Chang DT, Amdur RJ, Morris CG, et al. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys 2006;66:1051-5. [Crossref] [PubMed]
- Ang KK, Peters LJ, Weber RS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys 1994;30:795-8. [Crossref] [PubMed]
- Ballo MT, Ross MI, Cormier JN, et al. Combined-modality therapy for patients with regional nodal metastases from melanoma. Int J Radiat Oncol Biol Phys 2006;64:106-13. [Crossref] [PubMed]
- Stevens G, Thompson JF, Firth I, et al. Locally advanced melanoma: results of postoperative hypofractionated radiation therapy. Cancer 2000;88:88-94. [Crossref] [PubMed]
- Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, et al. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. Int J Radiat Oncol Biol Phys 1999;44:607-18. [Crossref] [PubMed]
- Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:809-16. [Crossref] [PubMed]
- Overgaard J, Overgaard M, Hansen PV, et al. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol 1986;5:183-92. [Crossref] [PubMed]
- Olivier KR, Schild SE, Morris CG, et al. A higher radiotherapy dose is associated with more durable palliation and longer survival in patients with metastatic melanoma. Cancer 2007;110:1791-5. [Crossref] [PubMed]


