Langerhans cell histiocytosis of skull: a retrospective study of 18 cases
Introduction
Langerhans cell histiocytosis (LCH) is a rare histiocytic disorder which affects all age groups with mostly unexplained etiology and unusual histology. Practically every organ can be involved by this disease, and the skull is a favored location. The clinical spectrum and prognosis of LCH are extremely diverse. Up to now, there is still no consensus exists for the optimal therapy for LCH, since this disorder was first described by Dr. Smith in 1865 (1). Meanwhile, there is also no generally accepted prognostic factor. The aims of this study were to evaluate our experience of LCH in Chinese patients with skull involvement, and also to investigate the related factors influencing the prognosis.
Methods
Patient selection and characteristics
We conducted a retrospective chart review of 18 consecutive patients treated at Sun Yat-sen University Cancer Center for LCH with the skull involvement between March 2002 and February 2014. The pathological diagnosis of LCH in each case was confirmed at the pathologists of the Sun Yat-sen University Cancer Center according to the World Health Organization classification of tumors (2). Clinical parameters of each case were checked, including patient demographics, location and amount of lesions, pathological profiles, and therapeutic modalities. Age, gender, extent of disease, type of therapy, reactivation, and long-term outcome were analyzed.
A complete work-up was performed for each case to determine the affected extent by LCH disease, including skull X-ray, head computed tomography (CT), chest X-ray, ultrasound of abdomen and pelvis regions. A magnetic resonance imaging (MRI) of brain was performed in 13 patients underwent to delineate the size and borders of the lesion in detail. Additionally, after the histopathologic diagnosis of LCH was confirmed, a skeletal survey had been performed using radionuclide bone scan.
In the present study, the patients of LCH without skull involvement were excluded. According to disease extent at diagnosis, the 18 LCH patients with skull involvement were divided into three groups: (I) unifocal-monosystem group, including ten cases with solitary skull lesion; (II) multifocal-monosystem group, including two cases with multiple bone lesions and no extra-skeletal involvement; (III) multisystem group, including six cases with LCH lesions involving both skeletal and extra-skeletal system. This study was approved by the ethics committee of Sun Yat-sen University Cancer Center.
Treatment
In unifocal-monosystem group, excision of the skull lesion was performed in eight of ten cases, a low dosage of local radiotherapy (20 Gy) and a purposeful observation was accept by the remaining two cases of this group after biopsy respectively. In multifocal-monosystem group, both of the two cases were received chemotherapy to control the multiple bone lesions, after their pathological diagnoses of LCH were confirmed by biopsy. In multi-system group, all the six cases were managed with systemic chemotherapy, after their diagnoses of LCH were confirmed by biopsy.
Results
A total of 18 patients, including 14 males and 4 females, with skull involvement of LCH were enrolled in the study. The mean age at the time of diagnosis was 9.4 years, ranged from 0.9 to 64 years. Follow-up duration ranged from 14 to 139 months, with a mean of 43.6 months. There was a male predominance in this disease, since the male/female ratio was 3.5:1. The patient’s age, sex, presentation, lesions location, treatment modalities and follow-up were summarized in Table 1.
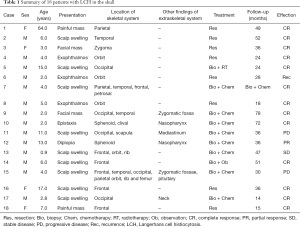
Full table
In our series, a skull mass with or without tenderness was the most common chief complaint (13 cases, 72.2%), followed by an exophthalmos (3 cases, 16.7%), diplopia (1 case, 5.6%), and epistaxis (1 case, 5.6%). Frontal bone was the most frequent affected locations of skull (6 cases, 33.3%), followed by occipital bone (5 cases, 27.8%), orbit bone (5 cases, 27.8%), temporal bone (4 cases, 22.2%), and parietal bone (3 cases, 16.7%). The involvement of basilar skull bone was observed in three cases, and the affected locations included sphenoid, clival and petrosal bone. The involvements of rib bone, scapula bone, femur bone, and sacral vertebrae were observed in our cases, but much less frequently than the skull bone. In the cases classified as multisystem LCH, the involvement of the tissues in zygomatic fossae, pituitary mediastinum and nasopharynx were recorded.
In our series, the clinical effect was evaluated with Macdonald standard. The classification of 18 patients and the efficacy of various treatments were summarized in Table 2. In unifocal-monosystem group, eight of ten were received surgical excision without other postoperative treatment, and seven of eight lesions remained free from LCH during the follow-up duration (ranged from 15 to 52 months, with a mean of 32.9 months). The remaining one lesion recurred 22 months after his surgical excision, and radiotherapy was performed to control the recurrent lesion. A complete response (CR) of lesion was also observed in the other two patients of this group, although they accept very different therapeutic regimen after biopsy, ranged from a junior dosage of radiotherapy (20 Gy) to a conservative observation. In multifocal-monosystem group, both of the two cases were received chemotherapy to control the multiple osseous lesions. A CR was obtained in one of them, after a chemotherapy protocol consisted of 12 cycles of vincaleukoblastine + 6-mercaptopurine + prednisone, and 6 cycles of vincristine + etoposide + prednisone, with a 139 months of follow-up. A stable disease (SD) of multiple osseous lesions was obtained in another case, after a chemotherapy protocol consisted of 15 cycles of vincaleukoblastine + 6-mercaptopurine + prednisone, and 9 cycles of vincaleukoblastine + 6-mercaptopurine + prednisone. In the six cases of multi-system group, the following chemotherapy regimens were applied, prednisone + etoposide + vincaleukoblastine, vincaleukoblastine + 6-mercaptopurine + prednisone, vincaleukoblastine + prednisone, and methotrexate + imuran + ornithine aspartate. After multi-cycles of chemotherapy, a CR in four cases and a partial response (PR) in one case were obtained, and a progressive disease (PD) was observed in the remaining one.
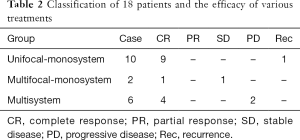
Full table
Discussion
Reclassification
Until the current reclassification of the disease by the Writing Group of the Histiocyte Society (3), LCH was formerly referred to as histiocytosis X (4), with three clinical subtypes, namely eosinophilic granuloma, Hand-Schûller-Christian’s disease, and Letterer-Siwe’s disease. Practically every organ can be involved by LCH, and significant overlapping has been observed studying the presentations of the three clinical subtypes mentioned above. In this study, the current reclassification was applied. In our series, the 18 LCH patients with skull involvements were firstly classified as 12 cases of monosystem form and 6 cases of multi-system form, and the 12 cases of mono-system form further subdivided into 10 cases of unifocal and 2 cases of multifocal types. In our opinion, the new classification is more convenient to evaluate the effectiveness of treatment and prognosis of LCH compared with the former one.
Clinical presentations
LCH arises from an abnormal proliferation of histiocytes, and most commonly presents as a solitary lesion in the skull, whereas femur, mandible, ribs, pelvis, and spine are other common locations (5-7). Our series consisted of 18 cases (14 males, 4 females; mean age, 9.4 years; range, 0.9 to 64 years), and there was an obvious male predominance (male/female ratio, 3.5:1). A skull mass with or without tenderness was the most common chief complaint (13 cases, 72.2%), and the frontal bone was the most frequent affected locations of skull (6 cases, 33.3%) in our series. In the skull, the typical appearance of a LCH lesion is a well-defined lytic lesion, with nonsclerotic margins, involving both inner and outer table, resulting in a double-contour appearance, sometimes associated with an adjacent soft tissue mass (Figure 1). A “button sequestrum” was once thought to be characteristic of skeletal LCH (Figure 2), but it may be seen in metastatic disease, radiation necrosis, dermoid and epidermoid cysts, fibrous dysplasia and meningioma (8,9).
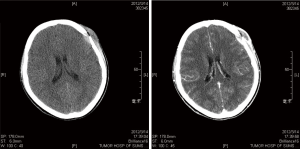
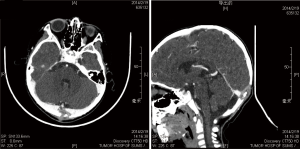
Treatment
All 18 patients were treated for their disease, and the choice of treatment was based on the disease extent. For the ten cases with unifocal lesion of skull, local resection, irradiation and observation had been utilized respectively. Systemic therapy had been used in the other eight cases with multifocal bone lesions or multisystem lesions. For the unifocal bone lesions, surgery resection is a typical treatment. Involvement of the skull base has been rarely reported. In our series, surgery resection was performed in eight unifocal skull lesions, including four involvements in calvarium bone and four involvements in the skull base. Complete remission was obtained in seven of eight, but one involvement in orbit recurred 24 months after surgery resection. It is clear that surgery resection is very effective for single skull bone lesions, but as a complex surgical area, the involvement affected in skull base was more challenging than that in calvarium bone. Though some authors recommend chemotherapy in case of involvement of the orbit and the temporal bone instead of surgical resection, according to our experience, based on the experienced surgeons and the choosing of optimal approach, surgery intervention is the mainstay treatment to unifocal skull bone lesion, and the approach should allow wide enough exposure of the tumor for achieving complete resection and manage the eventual complications (10). A low dosage of radiotherapy is also an effective treatment for the LCH lesions. In our series, a low dosage of radiotherapy allowed for a complete remission of an occipital lesion and a second time remission of recurrent orbital LCH lesions.
For the multifocal bone lesions or multisystem lesions of LCH, chemotherapy is an effective treatment. In our series, complete remission was obtained in five of eight patients who received multiple cycles of chemotherapy. Vinblastine and prednisone were reported as the standard treatment for children (11). But Cantu et al. reported that cytosine arabinoside (ARA-C) was an effective and minimally toxic treatment for LCH bone lesions in adults, and in contrast, vinblastine/prednisone results in poor overall responses and excessive toxicity (12). There were still very few publication literatures providing enough data on long term follow-up, toxicity data, or comparison of responses among various chemotherapy.
Spontaneous remission of LCH lesions has been reported previously (13,14). In our series, such spontaneous remission was also observed in a unifocal skull lesion with a follow-up of 51 months. De Angulo et al. reported eight cases of solitary calvarial LCH who were managed with observation, and only one required surgical intervention. They suggested a short period of observation may be useful in the initial management of solitary calvarial LCH lesion to avoid a surgical procedure (14). There is no enough publication literature on multicenter prospective trial to confirm these findings and determine guidelines or indications for surgery to manage these cases.
Conclusions
The unifocal-monosystem of LCH of the skull is a clinicopathological entity with a good outcome, and resection, irradiation or purposeful observation are also can be been utilized as the choice of treatment. For the multifocal bone lesions and multisystem lesions of LCH, chemotherapy is an effective treatment as a systemic therapy. There is no enough publication literature to determine guidelines or indications for managing this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Sun Yat-sen University Cancer Center and written informed consent was obtained from all patients.
References
- Smith T. Skull-cap showing congenital deficiencies of bone. Trans Path Soc Lond 1865;16:224-5.
- Jaffe R, Weiss LM, Facchetti F. Tumors derived from Langerhans cells. In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon: IARC Press, 2008:358-60.
- Histiocytosis syndromes in children. Writing group of the Histiocyte Society. Lancet 1987;1:208-9. [PubMed]
- Lichtenstein L. Histiocytosis X; integration of eosinophilic granuloma of bone, Letterer-Siwe disease, and Schüller-Christian disease as related manifestations of a single nosologic entity. AMA Arch Pathol 1953;56:84-102. [PubMed]
- Hoover KB, Rosenthal DI, Mankin H. Langerhans cell histiocytosis. Skeletal Radiol 2007;36:95-104. [Crossref] [PubMed]
- Kamimura M, Kinoshita T, Itoh H, et al. Eosinophilic granuloma of the spine: early spontaneous disappearance of tumor detected on magnetic resonance imaging. Case report. J Neurosurg 2000;93:312-6. [PubMed]
- Park SH, Park J, Hwang JH, et al. Eosinophilic granuloma of the skull: a retrospective analysis. Pediatr Neurosurg 2007;43:97-101. [Crossref] [PubMed]
- Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics 1992;12:801-23. [Crossref] [PubMed]
- Rawlings CE 3rd, Wilkins RH. Solitary eosinophilic granuloma of the skull. Neurosurgery 1984;15:155-61. [Crossref] [PubMed]
- López F, Llorente JL, Suárez C. Surgical treatment of eosinophilic granuloma of the infratemporal fossa: a successful treatment option. Acta Otolaryngol 2012;132:558-62. [Crossref] [PubMed]
- Gadner H, Grois N, Pötschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood 2008;111:2556-62. [Crossref] [PubMed]
- Cantu MA, Lupo PJ, Bilgi M, et al. Optimal therapy for adults with Langerhans cell histiocytosis bone lesions. PLoS One 2012;7:e43257. [Crossref] [PubMed]
- Oliveira M, Steinbok P, Wu J, et al. Spontaneous resolution of calvarial eosinophilic granuloma in children. Pediatr Neurosurg 2003;38:247-52. [Crossref] [PubMed]
- De Angulo G, Nair S, Lee V, et al. Nonoperative management of solitary eosinophilic granulomas of the calvaria. J Neurosurg Pediatr 2013;12:1-5. [Crossref] [PubMed]


